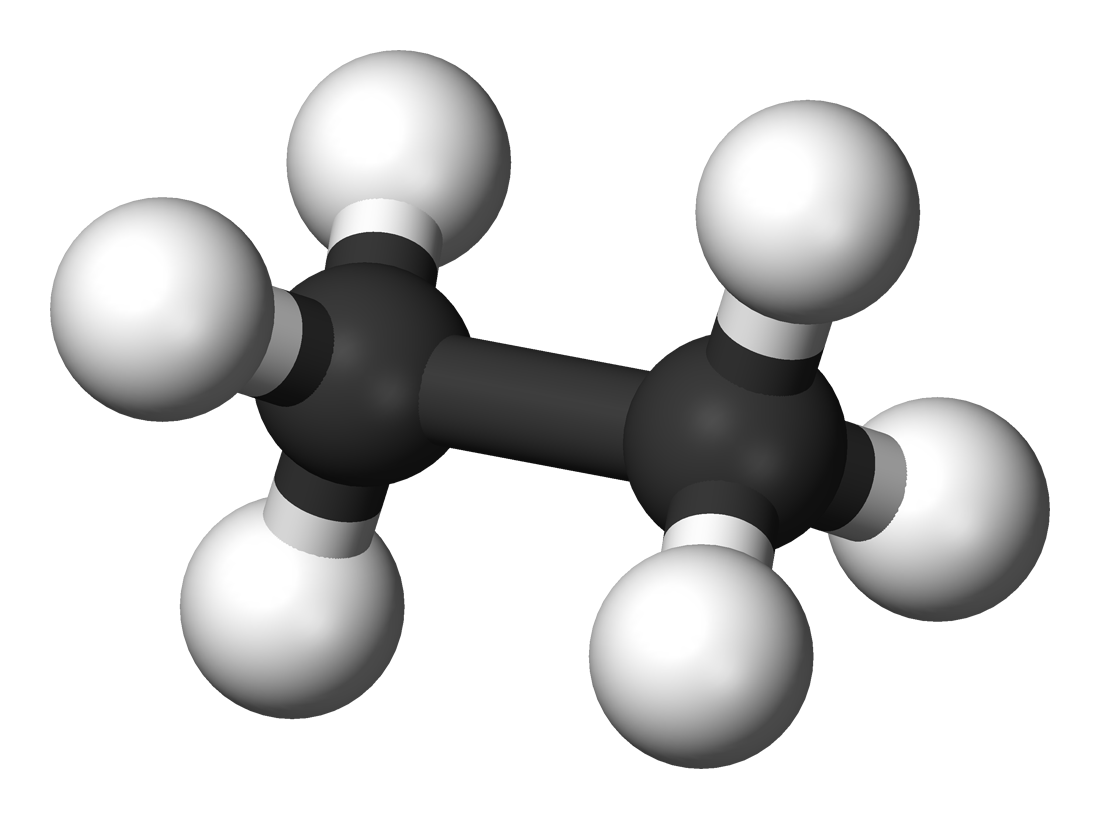
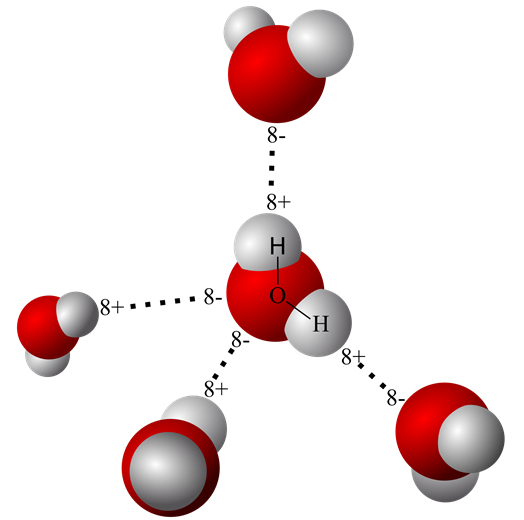
Will Ethane Form A Hydrogen Bond With Water
Will Ethane Form A Hydrogen Bond With Water - It has the chemical formula ch. • broad range of geometric hydrogen bond definitions is. In h 2 o, only two of the six. Web the chemical formula of ethane? Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. The methane molecule provides an example: Web ethylene/ethane separation is validated by breakthrough experiments with high purity of ethylene (99.1%) at 333 k. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web other common substances which are freely soluble in water because they can hydrogen bond with water molecules include ethanol (alcohol) and sucrose. We investigated factors that could affect the hydrogen.
Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. We investigated factors that could affect the hydrogen. It has the chemical formula ch. What is the intramolecular bond that holds the hydrogen and carbon atoms within an ethane molecule? Is an ethane molecule a. Web ethylene/ethane separation is validated by breakthrough experiments with high purity of ethylene (99.1%) at 333 k. Web ethoxyethane, better known as diethyl ether or even just ether, can form hydrogen bonds with water. The two carbon atoms bond by merging their remaining sp 3. Oxygen is highly electronegative, which creates a partial negative charge on one end of. • broad range of geometric hydrogen bond definitions is.
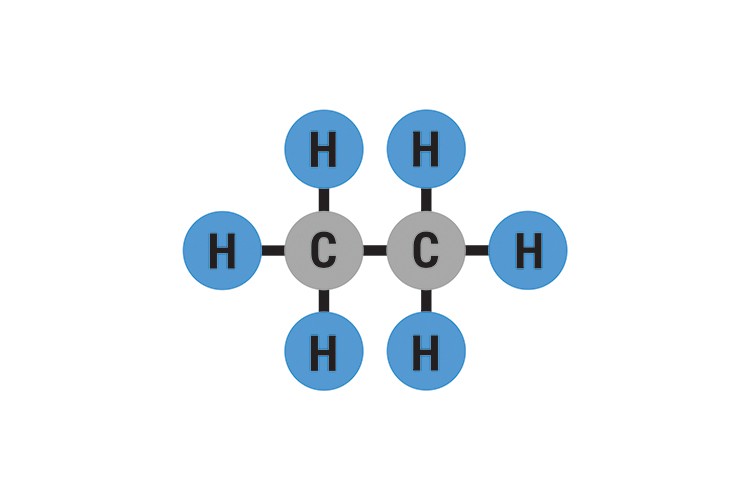
No ethane (c2h6) is a hydrocarbon and hydrocarbons can't form hydrogen bonds. Web will ethane form a hydrogen bond with water? Web the hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. In h 2 o, only two of the six. We investigated factors that could affect the hydrogen. But fitting the ether molecules into the water solvent. • broad range of geometric hydrogen bond definitions is. Web ethane + oxygen → carbon dioxide + water. Oxygen is highly electronegative, which creates a partial negative charge on one end of. Web the chemical formula of ethane?
What is a hydrogen bond? [With free chemistry study guide]
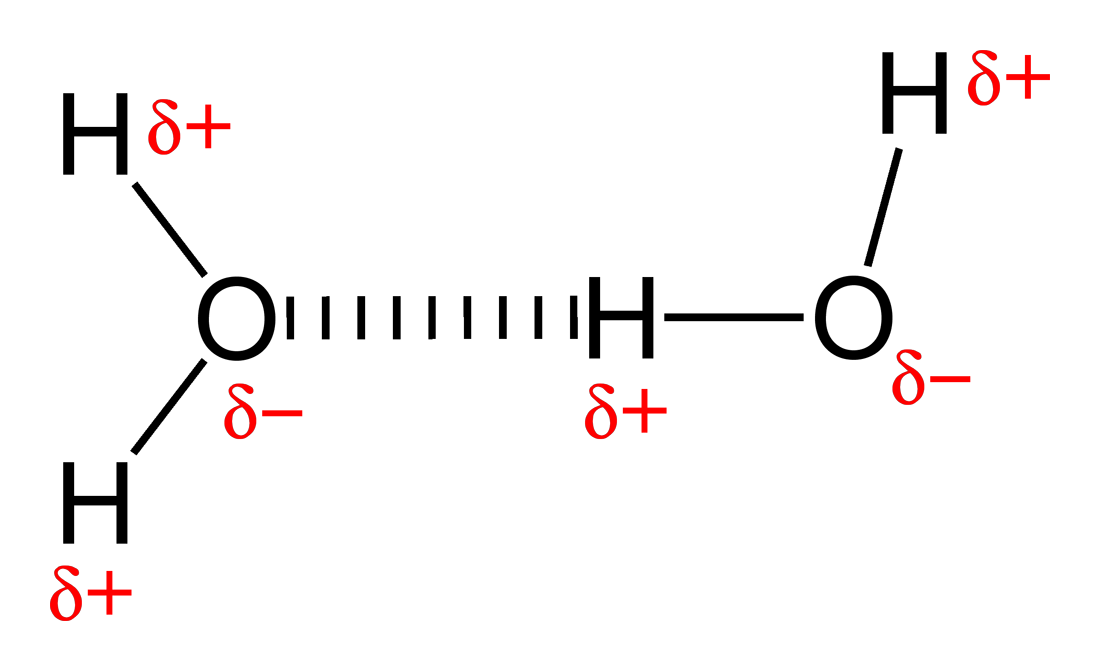
In h 2 o, only two of the six. Web ethane, {eq}\textrm{c}_2\textrm{h}_6 {/eq}, will not form hydrogen bonds with water, {eq}\textrm{h}_2\textrm{o} {/eq}, because ethane is nonpolar. The two carbon atoms bond by merging their remaining sp 3. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. But fitting the ether.
The Curious Wavefunction A bond by any other name... How the simple
In h 2 o, only two of the six. What is the intramolecular bond that holds the hydrogen and carbon atoms within an ethane molecule? We investigated factors that could affect the hydrogen. Web will ethane form a hydrogen bond with water? It has the chemical formula ch.
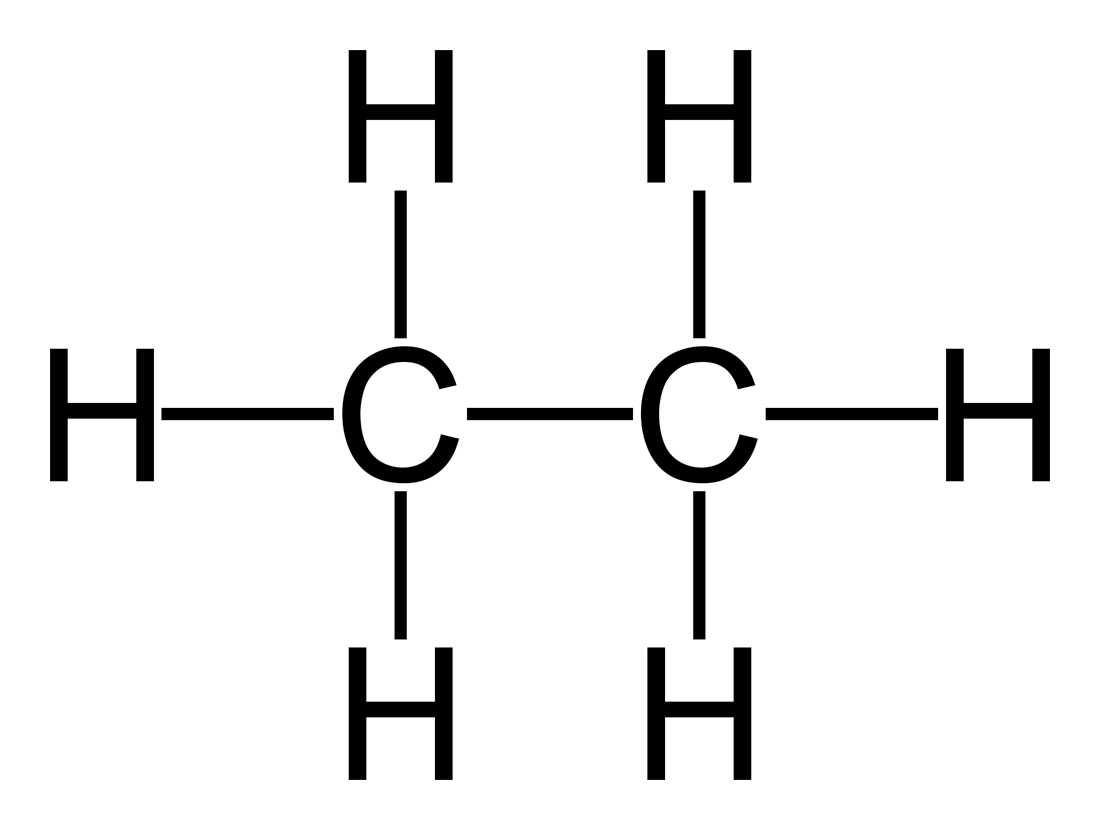
The molecular structure of Ethane and formula structure
What is the intramolecular bond that holds the hydrogen and carbon atoms within an ethane molecule? Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Web ethoxyethane, better known as diethyl ether or even just ether, can form hydrogen bonds with water. Web therefore, carbon atoms can form up to four covalent.
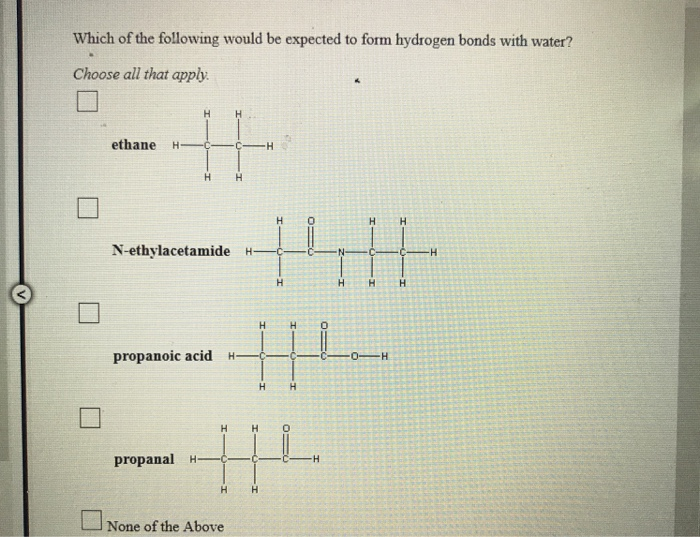
Solved Which of the following would be expected to form
Web the chemical formula of ethane? What is the intramolecular bond that holds the hydrogen and carbon atoms within an ethane molecule? No ethane (c2h6) is a hydrocarbon and hydrocarbons can't form hydrogen bonds. But fitting the ether molecules into the water solvent. The two carbon atoms bond by merging their remaining sp 3.
Pin on Hydrogen
Web will ethane form a hydrogen bond with water? We investigated factors that could affect the hydrogen. The two carbon atoms bond by merging their remaining sp 3. C2h4(g) + 3o2(g) → 2co2(g) + 2h2o (l). Web ethane + oxygen → carbon dioxide + water.
Solved Below are three molecules, hexan3one,
Web other common substances which are freely soluble in water because they can hydrogen bond with water molecules include ethanol (alcohol) and sucrose. Web ethane + oxygen → carbon dioxide + water. Web will ethane form a hydrogen bond with water? The methane molecule provides an example: Web therefore, carbon atoms can form up to four covalent bonds with other.
8.1 Intermolecular Interactions The Basics of General, Organic, and
Web other common substances which are freely soluble in water because they can hydrogen bond with water molecules include ethanol (alcohol) and sucrose. Web the hydrogens bond with the two carbons to produce molecular orbitals just as they did with methane. In h 2 o, only two of the six. The methane molecule provides an example: Web ethylene/ethane separation is.
How many covalent bonds are there in a molecule of class 12 chemistry CBSE
But fitting the ether molecules into the water solvent. Is an ethane molecule a. Web other common substances which are freely soluble in water because they can hydrogen bond with water molecules include ethanol (alcohol) and sucrose. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. Oxygen is highly electronegative,.
Article 167 The Geometry of Molecules Part 2 Visual Library
The two carbon atoms bond by merging their remaining sp 3. Web will ethane form a hydrogen bond with water? Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. • broad range of geometric hydrogen bond definitions is. Web ethane, {eq}\textrm{c}_2\textrm{h}_6 {/eq}, will not form hydrogen bonds with water, {eq}\textrm{h}_2\textrm{o}.
hydrophilic vs. hydrophobic
Web ethane + oxygen → carbon dioxide + water. In h 2 o, only two of the six. • broad range of geometric hydrogen bond definitions is. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule. What is the intramolecular bond that holds the hydrogen and carbon atoms within an.
Web Same Thing With This One, Once It Vaporizes, It's Out In Gaseous State, It's Much Further From Any Other Water Molecules, It's Not Going To Be Able To Form Those Hydrogen Bonds With.
Web other common substances which are freely soluble in water because they can hydrogen bond with water molecules include ethanol (alcohol) and sucrose. We investigated factors that could affect the hydrogen. Web ethoxyethane, better known as diethyl ether or even just ether, can form hydrogen bonds with water. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape.
It Has The Chemical Formula Ch.
Web ethane + oxygen → carbon dioxide + water. What is the intramolecular bond that holds the hydrogen and carbon atoms within an ethane molecule? C2h6(g) + 3½o2(g) → 2co2(g) + 3h2o (l) ethene + oxygen → carbon dioxide + water. But fitting the ether molecules into the water solvent.
Web The Hydrogens Bond With The Two Carbons To Produce Molecular Orbitals Just As They Did With Methane.
No ethane (c2h6) is a hydrocarbon and hydrocarbons can't form hydrogen bonds. Web ethylene/ethane separation is validated by breakthrough experiments with high purity of ethylene (99.1%) at 333 k. Is an ethane molecule a. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to satisfy the octet rule.
Web In Water, Each Hydrogen Nucleus Is Covalently Bound To The Central Oxygen Atom By A Pair Of Electrons That Are Shared Between Them.
C2h4(g) + 3o2(g) → 2co2(g) + 2h2o (l). Web will ethane form a hydrogen bond with water? The two carbon atoms bond by merging their remaining sp 3. The methane molecule provides an example:
![What is a hydrogen bond? [With free chemistry study guide]](http://www.aceorganicchem.com/blog/wp-content/uploads/2018/04/4-22-2018-6-20-10-PM.jpg)