Why Do Nonmetals Tend To Form Anions
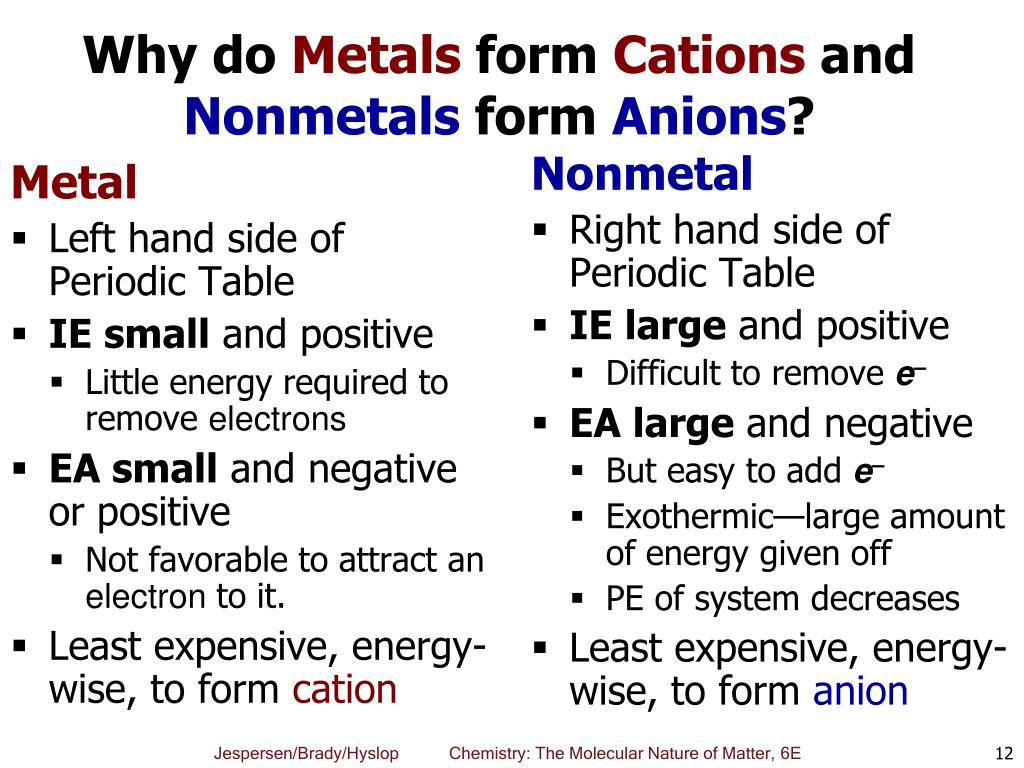
Why Do Nonmetals Tend To Form Anions - Web view the full answer. Web most nonmetals become anions when they make ionic compounds. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Web why do nonmetals and halogens tend to become anions? Web (i) atomic radii decrease across the period from left to right, and (ii) metals tend to form cations, and (iii), in answer to your specific question, non metals tend to. Web 1 2 o2 +2h + +2e− → h 2o ;e∘ = 1.23v. A neutral chlorine atom has seven electrons in its outermost shell. These are electronegative elements with high ionization energies. Web whether metals or non metals, the rule is that the element wants to reach the electron configuration of the nearest noble gas, the easiest way possible. Solution verified create an account to view solutions recommended textbook solutions.
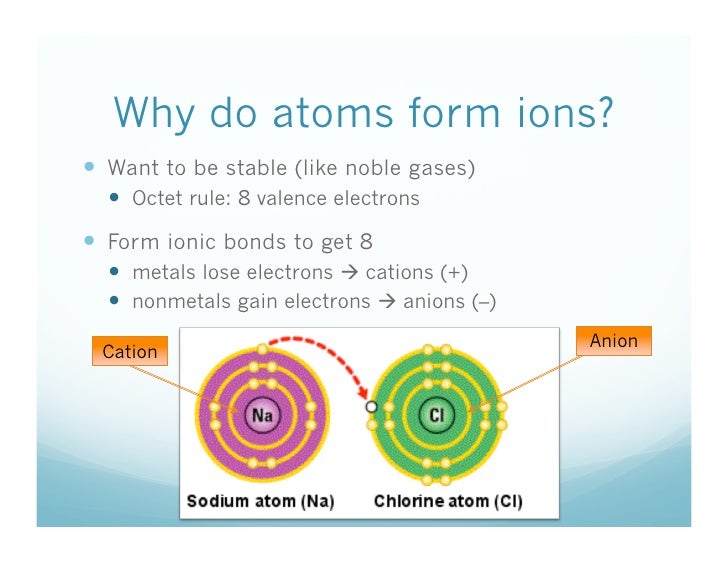
It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Solution verified create an account to view solutions recommended textbook solutions. Web why do nonmetals and halogens tend to become anions? Web nonmetals form negatively charged ions, or anions. Web whether metals or non metals, the rule is that the element wants to reach the electron configuration of the nearest noble gas, the easiest way possible. Web 1 2 o2 +2h + +2e− → h 2o ;e∘ = 1.23v. They do this because they need to gain one to three electrons in order to achieve an octet of valence electrons ,. Web view the full answer. Energy is released when electrons are removed from metal ions. These are electronegative elements with high ionization energies.
Web why do nonmetal atoms form anions when they react to form compounds? It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Web most nonmetals become anions when they make ionic compounds. Aniseed may 11, 2020 may 11, 2020 #1 aniseed 8 0 tl;dr summary generally speaking the elements in. These are electronegative elements with high ionization energies. Web view the full answer. Web nonmetals form negatively charged ions, or anions. Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound. Web 1 2 o2 +2h + +2e− → h 2o ;e∘ = 1.23v. They do this because they need to gain one to three electrons in order to achieve an octet of valence electrons ,.
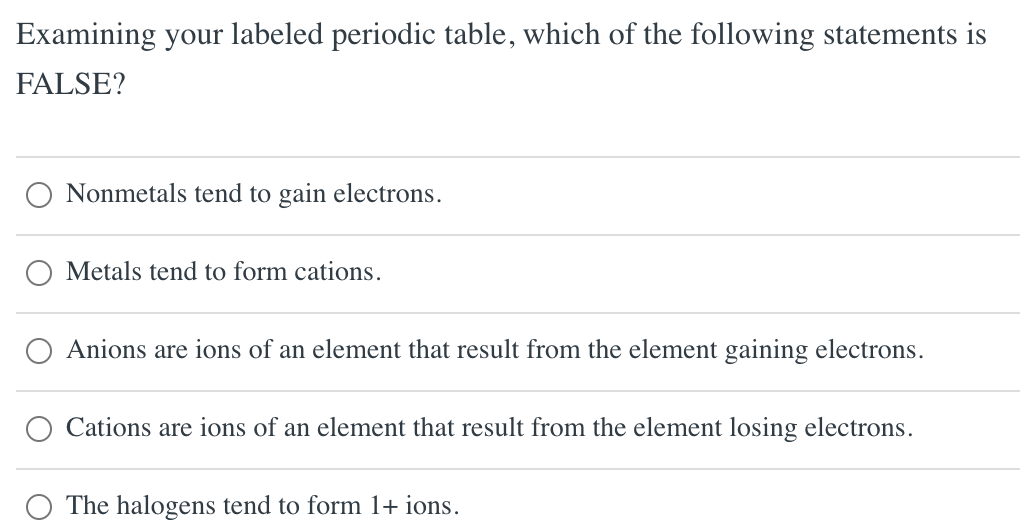
Halogens tend to form anions because A) losing
Web why do nonmetal atoms form anions when they react to form compounds? Web chemistry question why do nonmetals tend to form anions rather than cations? Web view the full answer. Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound. Energy is released when electrons are removed.
is incorreer? all s area eleseis erand e are metals b, all p area
Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound. Aniseed may 11, 2020 may 11, 2020 #1 aniseed 8 0 tl;dr summary generally speaking the elements in. Energy is released when electrons are removed from metal ions. Solution verified create an account to view solutions recommended textbook.
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
These are electronegative elements with high ionization energies. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Solution verified create an account to view solutions recommended textbook solutions. Web view the full answer. It is easiest to achieve noble gas configurations by removing valence electrons from metals.
Nonmetals and anion formation YouTube
Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Solution verified create an account to view solutions recommended textbook solutions. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Energy is released when electrons are removed from metal ions..
Nonmetals on Periodic Table JulietatWaller
Web nonmetals form negatively charged ions, or anions. Solution verified create an account to view solutions recommended textbook solutions. Web why do nonmetals and halogens tend to become anions? Aniseed may 11, 2020 may 11, 2020 #1 aniseed 8 0 tl;dr summary generally speaking the elements in. Identify the reason why metals tend to form cations and nonmetals tend to.
Solved Using your labeled periodic table, predict the charge
Web why do nonmetal atoms form anions when they react to form compounds? Energy is released when electrons are removed from metal ions. They do this because they need to gain one to three electrons in order to achieve an octet of valence electrons ,. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which.
Solved Pls Answer This Question. Thanks. What's The Form...
A neutral chlorine atom has seven electrons in its outermost shell. Web whether metals or non metals, the rule is that the element wants to reach the electron configuration of the nearest noble gas, the easiest way possible. Aniseed may 11, 2020 may 11, 2020 #1 aniseed 8 0 tl;dr summary generally speaking the elements in. Identify the reason why.
10. List at least three examples of pairs... Physical Chemistry
These are electronegative elements with high ionization energies. Web why do nonmetals and halogens tend to become anions? It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Web 1 2 o2 +2h + +2e− → h 2o ;e∘ = 1.23v. A neutral chlorine atom has seven electrons in its outermost shell.
10 28 How Many Electrons Do Atoms Gain Lose
Web (i) atomic radii decrease across the period from left to right, and (ii) metals tend to form cations, and (iii), in answer to your specific question, non metals tend to. Aniseed may 11, 2020 may 11, 2020 #1 aniseed 8 0 tl;dr summary generally speaking the elements in. These are electronegative elements with high ionization energies. Web chemistry question.
Solved Question 6 (1 point) Why do metals tend to form
Web why do nonmetal atoms form anions when they react to form compounds? Web (i) atomic radii decrease across the period from left to right, and (ii) metals tend to form cations, and (iii), in answer to your specific question, non metals tend to. Web 1 2 o2 +2h + +2e− → h 2o ;e∘ = 1.23v. Web view the.
Energy Is Released When Electrons Are Removed From Metal Ions.
Solution verified create an account to view solutions recommended textbook solutions. They do this because they need to gain one to three electrons in order to achieve an octet of valence electrons ,. Web when nonmetals gain electrons, the energy change is usually negative because they give off energy to form an anion (exothermic process); Web chemistry question why do nonmetals tend to form anions rather than cations?
A Neutral Chlorine Atom Has Seven Electrons In Its Outermost Shell.
Web whether metals or non metals, the rule is that the element wants to reach the electron configuration of the nearest noble gas, the easiest way possible. It is easiest to achieve noble gas configurations by removing valence electrons from metals (which have few. Web why do nonmetals and halogens tend to become anions? These are electronegative elements with high ionization energies.
Web View The Full Answer.
Web most nonmetals become anions when they make ionic compounds. Web (i) atomic radii decrease across the period from left to right, and (ii) metals tend to form cations, and (iii), in answer to your specific question, non metals tend to. Web nonmetals form negatively charged ions, or anions. Aniseed may 11, 2020 may 11, 2020 #1 aniseed 8 0 tl;dr summary generally speaking the elements in.
Web Why Do Nonmetal Atoms Form Anions When They React To Form Compounds?
Web 1 2 o2 +2h + +2e− → h 2o ;e∘ = 1.23v. Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound.