Why Do Atoms Form Bond
Why Do Atoms Form Bond - Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. Scishow explains what makes atoms bond (and what makes them sometimes seem promiscuous). We can therefore say that a molecule is the simplest unit of a covalent. Examples of these are diatomic oxygen (double bond) or nitrogen (triple bond). Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. Web more than one bond can be formed between atoms leading to double and triple bonds. Web when atoms combine by forming covalent bonds, the resulting collection of atoms is called a molecule. Some of the attractive forces are. The combination of multiple atoms, or chemical bonding, forms molecules. See how they're represented by.
Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. Web when atoms combine by forming covalent bonds, the resulting collection of atoms is called a molecule. Web atoms form bonds to try to achieve the same electron configuration as the noble gases. Atoms with a full valence electron orbital are less reactive. Scishow explains what makes atoms bond (and what makes them sometimes seem promiscuous). Web when atoms of two or more elements bond together, they make a compound. Web more than one bond can be formed between atoms leading to double and triple bonds. Web why do atoms form chemical bonds? See how they're represented by. Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms.
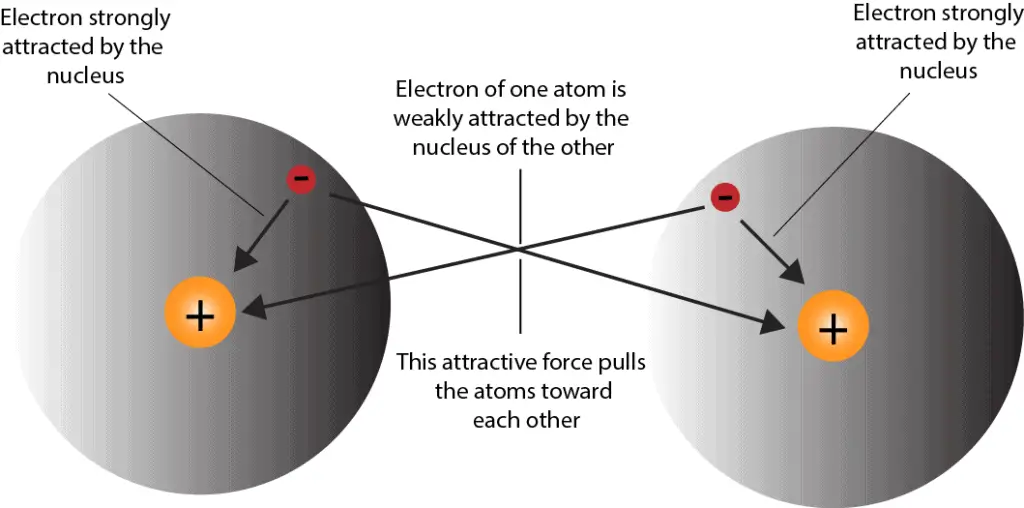
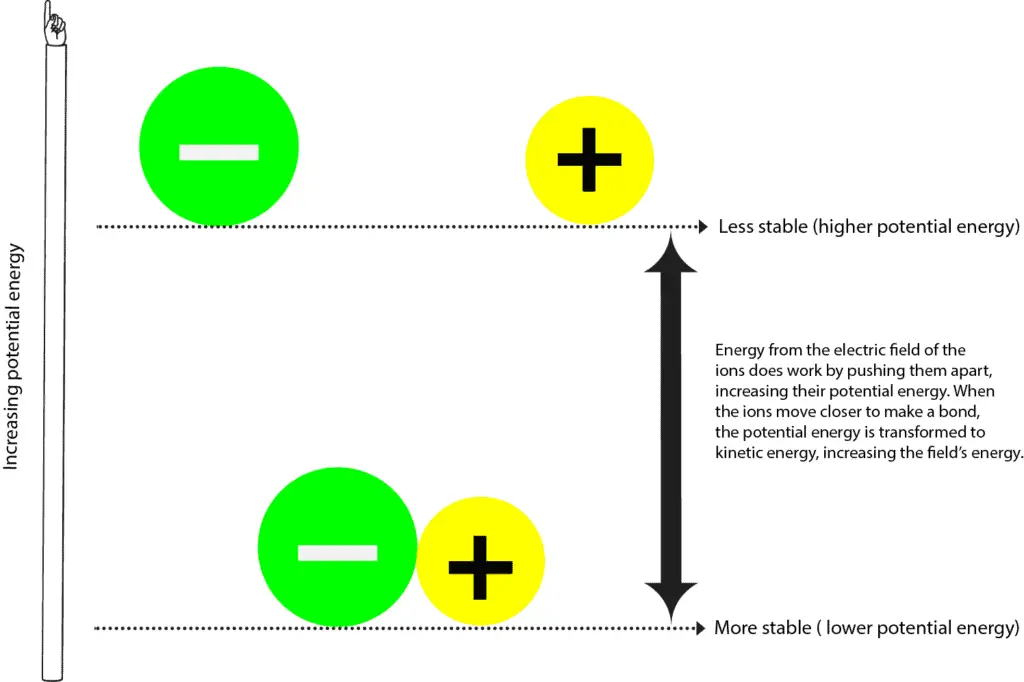
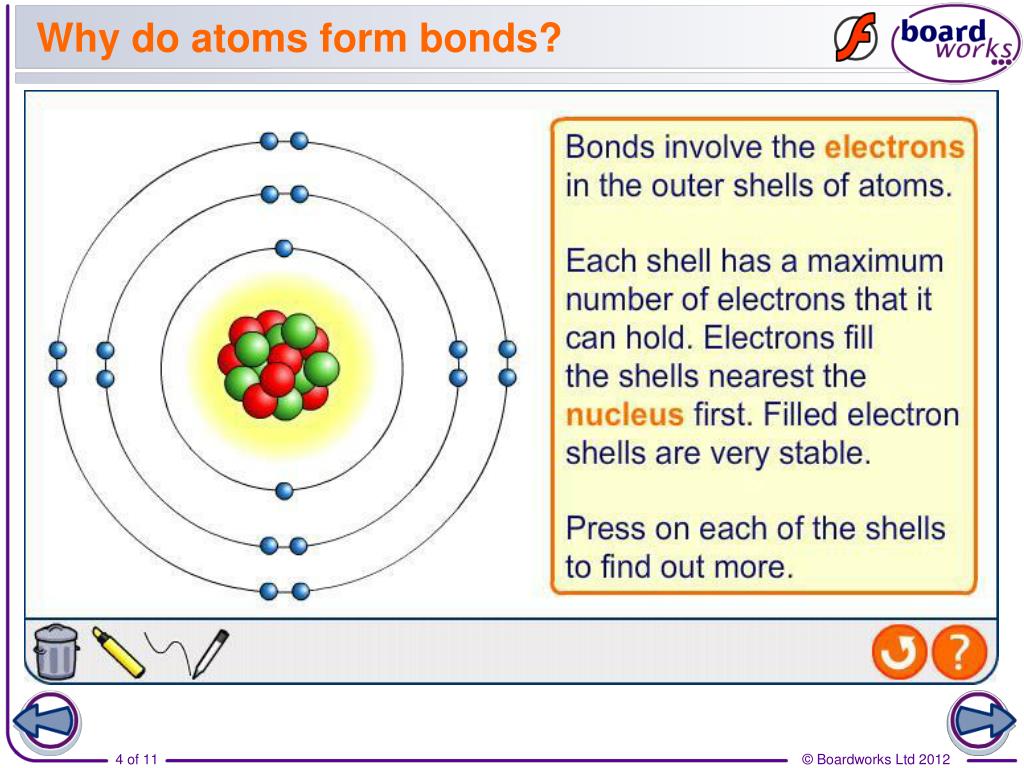
Atoms with a full valence electron orbital are less reactive. Examples of these are diatomic oxygen (double bond) or nitrogen (triple bond). The more “crowded” a given space is with atoms, the more likely it is that. Web why do atoms form chemical bonds? So, in order to fill. Web the summary of the answer is that the amount of energy involved in the electromagnetic field of two isolated hydrogen atoms is a lot more than the energy. With the exception of the noble gases, the other elements do not have completely filled valence shells (the outermost energy shell). Some of the attractive forces are. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. Click the card to flip 👆 atoms form chemical bonds because they want a lower energy and more stable electron configuration.
Why Do Atoms Form Chemical Bond in Urdu Hindi Lecture /Chemistry for
Web when atoms of two or more elements bond together, they make a compound. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. The combination of multiple atoms, or chemical bonding, forms molecules. Web why do atoms form chemical bonds? So, in order to fill.
How do atoms form covalent bond?
Web why do atoms form chemical bonds? Web atoms are individual units made up of protons, neutrons, and electrons. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. There are several types.
Why do atoms form bonds? YouTube
Web when atoms of two or more elements bond together, they make a compound. Chemical bonds are formed when electrons in different atoms. Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. In fact, they have to collide (bump together). Web atoms are individual units made up of protons, neutrons,.
Class 9 Chemistry Chapter 4 Lecture 8 Introduction Why do atoms form
Web atoms form bonds to try to achieve the same electron configuration as the noble gases. The combination of multiple atoms, or chemical bonding, forms molecules. Web atoms are individual units made up of protons, neutrons, and electrons. The more “crowded” a given space is with atoms, the more likely it is that. We can therefore say that a molecule.
Why Do Atoms Bond? YouTube
Some of the attractive forces are. Web when atoms of two or more elements bond together, they make a compound. Atoms with a full valence electron orbital are less reactive. Web why do atoms form chemical bonds? Examples of these are diatomic oxygen (double bond) or nitrogen (triple bond).
Why do atoms bond YouTube
With the exception of the noble gases, the other elements do not have completely filled valence shells (the outermost energy shell). Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. See how they're represented by. Click the card to flip 👆 atoms form chemical bonds because they want a lower.
Why do atoms bond with one another?
Scishow explains what makes atoms bond (and what makes them sometimes seem promiscuous). Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. Web why do atoms form chemical bonds? See how they're represented by. The combination of multiple atoms, or chemical bonding, forms molecules.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
The more “crowded” a given space is with atoms, the more likely it is that. Web when atoms of two or more elements bond together, they make a compound. Web atoms are individual units made up of protons, neutrons, and electrons. So, in order to fill. Web atoms form bonds to try to achieve the same electron configuration as the.
PPT Ions PowerPoint Presentation, free download ID6738771
We can therefore say that a molecule is the simplest unit of a covalent. Web atoms are individual units made up of protons, neutrons, and electrons. Web why do atoms form chemical bonds? The more “crowded” a given space is with atoms, the more likely it is that. With the exception of the noble gases, the other elements do not.
PPT Why do atoms bond? PowerPoint Presentation, free download ID
The combination of multiple atoms, or chemical bonding, forms molecules. Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. Atoms with a full valence electron orbital are less reactive. Web atoms are individual units made up of protons, neutrons, and electrons. Examples of these are diatomic oxygen (double bond) or.
Examples Of These Are Diatomic Oxygen (Double Bond) Or Nitrogen (Triple Bond).
Atoms with a full valence electron orbital are less reactive. These clusters of strongly bonded atoms are called molecules. Click the card to flip 👆 atoms form chemical bonds because they want a lower energy and more stable electron configuration. Web atoms have to be close together to form a bond.
Scishow Explains What Makes Atoms Bond (And What Makes Them Sometimes Seem Promiscuous).
Chemical bonds are formed when electrons in different atoms. Web atoms are individual units made up of protons, neutrons, and electrons. Some of the attractive forces are. We can therefore say that a molecule is the simplest unit of a covalent.
Web Atoms Form Bonds To Try To Achieve The Same Electron Configuration As The Noble Gases.
See how they're represented by. The more “crowded” a given space is with atoms, the more likely it is that. There are several types of bond. Web atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms.
Web When Atoms Combine By Forming Covalent Bonds, The Resulting Collection Of Atoms Is Called A Molecule.
Web more than one bond can be formed between atoms leading to double and triple bonds. Attractive forces between atoms that are strong enough to make the linked elements function as a single unit. Web when atoms of two or more elements bond together, they make a compound. In fact, they have to collide (bump together).