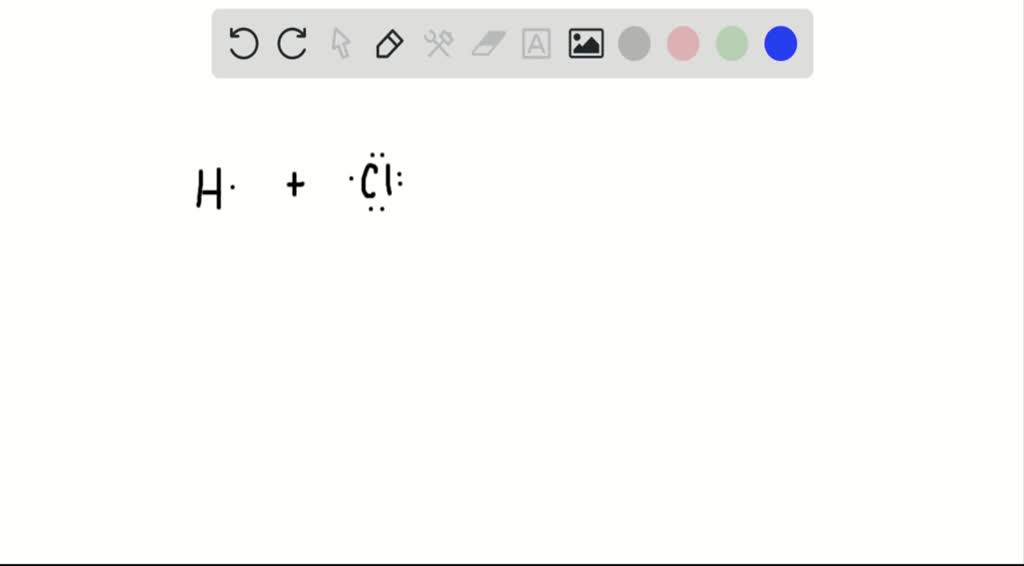
Why Do Atoms Combine To Form Molecules
Why Do Atoms Combine To Form Molecules - When two atoms share electrons. Web why do atoms combine to form molecules. Web why form chemical bonds? Web atoms come together to form molecules because of their electrons. Web atoms combine in a ratio of small whole numbers to form compounds. Last updated at may 29, 2023 by teachoo. Question why do atoms combine? Solution the atoms combine to attain a noble or inert gas electronic configuration, in order to. Web they form the exterior of an atom and specify the rules of chemistry by which atoms combine to form molecules. Where does the valence electrons exist in an atom?
Last updated at may 29, 2023 by teachoo. Web atoms combine in a ratio of small whole numbers to form compounds. It has basic structural and fundamental units. Web best answer copy two atoms [one superficient of an electron and one deficient of an electron] form the chemical bond by the sharing of one pair of electrons. Web why do atoms combine to form molecules atoms are smallest units of matter, most of these atoms other than atoms of noble or inert gases are less stable which means they great. Web why form chemical bonds? But they cannot exist independently. Electrons can join (or bond ) atoms together in two main ways. Many atoms become stable when their. Web expert answer 100% (4 ratings) transcribed image text:
Web why are atoms prevalent mostly as molecules? Web if you learned in chemistry that some atoms tend to gain or lose electrons or form bonds with each other, those facts remain true even when the atoms or molecules are part of a living. Question why do atoms combine? Electrons can join (or bond ) atoms together in two main ways. Web chemistry octet rule why do atoms. Last updated at may 29, 2023 by teachoo. Many atoms become stable when their. Electrons are in continuous motion around the nucleus , but. Why do atoms combine to form molecules? Web atoms combine in a ratio of small whole numbers to form compounds.
why do atoms react why do atoms react with one another to form
Many atoms become stable when their. Web they form the exterior of an atom and specify the rules of chemistry by which atoms combine to form molecules. Where does the valence electrons exist in an atom? Web expert answer 100% (4 ratings) transcribed image text: The matter is anything and everything in our surrounding.
Why Do Atoms Form Molecules? Wonderopolis
All natural systems tend to adopt a state of lowest. Atoms are the smallest particles that can exist in nature. Web if you learned in chemistry that some atoms tend to gain or lose electrons or form bonds with each other, those facts remain true even when the atoms or molecules are part of a living. Atoms are smallest units.
PPT What are bonds? PowerPoint Presentation, free download ID5980343
Last updated at may 29, 2023 by teachoo. Web chemistry octet rule why do atoms. Electrons can join (or bond ) atoms together in two main ways. Question why do atoms combine? But they cannot exist independently.
Why do atoms combine to form molecule ? YouTube
The matter is anything and everything in our surrounding. Web atoms combine in a ratio of small whole numbers to form compounds. It has basic structural and fundamental units. Web best answer copy two atoms [one superficient of an electron and one deficient of an electron] form the chemical bond by the sharing of one pair of electrons. Web atoms.
Why do atoms form molecules? Quantum mechanical model of atoms. Arvin
Solution the atoms combine to attain a noble or inert gas electronic configuration, in order to. Web expert answer 100% (4 ratings) transcribed image text: Last updated at may 29, 2023 by teachoo. Question why do atoms combine? The key to understanding why atoms link together is energy.
Why do atoms combine to form molecules SolvedLib
All natural systems tend to adopt a state of lowest. Many atoms become stable when their. Web why form chemical bonds? The matter is anything and everything in our surrounding. Solution the atoms combine to attain a noble or inert gas electronic configuration, in order to.
Bonding How Atoms Combine
Web why do atoms combine to form molecules. Last updated at may 29, 2023 by teachoo. Web why do atoms combine to form molecules atoms are smallest units of matter, most of these atoms other than atoms of noble or inert gases are less stable which means they great. But they cannot exist independently. Web they form the exterior of.
Why do atoms combine to form a molecule
Many atoms become stable when their. Electrons can join (or bond ) atoms together in two main ways. Web why do atoms combine to form molecules. Question why do atoms combine? When two atoms share electrons.
Why Do Atoms Form Molecules? Wonderopolis
Web atoms combine in a ratio of small whole numbers to form compounds. Solution the atoms combine to attain a noble or inert gas electronic configuration, in order to. Web why do atoms combine? All natural systems tend to adopt a state of lowest. Web atoms come together to form molecules because of their electrons.
How Do Atoms Come Together to Form Molecules? Sciencing
Web why form chemical bonds? Web why are atoms prevalent mostly as molecules? Web they form the exterior of an atom and specify the rules of chemistry by which atoms combine to form molecules. Web chemistry octet rule why do atoms. Question why do atoms combine?
Web Atoms Combine In A Ratio Of Small Whole Numbers To Form Compounds.
Web chemistry octet rule why do atoms. Question why do atoms combine? Web best answer copy two atoms [one superficient of an electron and one deficient of an electron] form the chemical bond by the sharing of one pair of electrons. All natural systems tend to adopt a state of lowest.
Atoms Are Smallest Units Of Matter, Most Of These Atoms Other Than Atoms Of Noble Or Inert Gases Are Less Stable Which.
Electrons are in continuous motion around the nucleus , but. Many atoms become stable when their. Web why form chemical bonds? Web if you learned in chemistry that some atoms tend to gain or lose electrons or form bonds with each other, those facts remain true even when the atoms or molecules are part of a living.
Where Does The Valence Electrons Exist In An Atom?
It has basic structural and fundamental units. Why do atoms combine to form molecules? Web why do atoms combine to form molecules atoms are smallest units of matter, most of these atoms other than atoms of noble or inert gases are less stable which means they great. Web they form the exterior of an atom and specify the rules of chemistry by which atoms combine to form molecules.
The Key To Understanding Why Atoms Link Together Is Energy.
The matter is anything and everything in our surrounding. Solution the atoms combine to attain a noble or inert gas electronic configuration, in order to. Atoms are the smallest particles that can exist in nature. Web expert answer 100% (4 ratings) transcribed image text: