What Is The Proper Form Of The Combined Gas Law
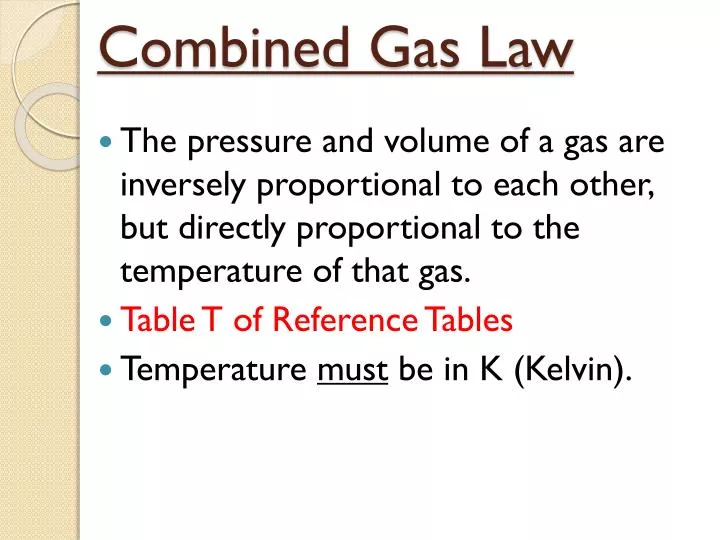
What Is The Proper Form Of The Combined Gas Law - In order to compute the changes in temperature, pressure or. Web this gas law is known as the combined gas law, and its mathematical form is. P is the pressure of the gas. Web if the number of moles of an ideal gas are kept constant under two different sets of conditions, a useful mathematical relationship called the combined gas law is. Web combined gas law formula. Combined gas law can be mathematically expressed as. What is the proper form of the combined gas law? T is the temperature of the gas. Web the most common form of the equation for the combined gas law is as follows: This allows us to follow changes in all three.
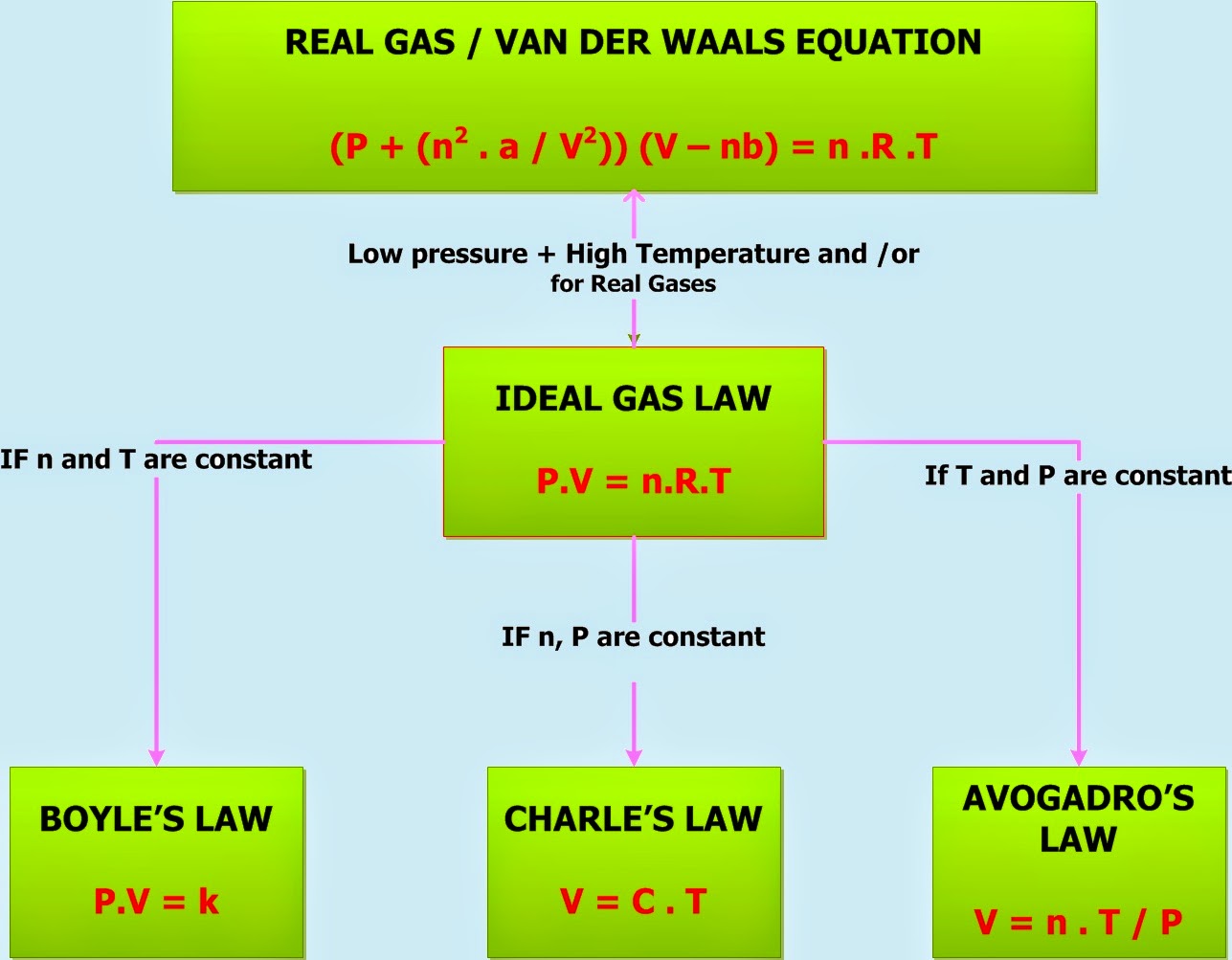
P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the. In order to compute the changes in temperature, pressure or. Web combined gas law formula: Web the combined gas law is a formula about ideal gases. Combined gas law can be mathematically expressed as. What is the proper form of the combined gas law? Web the combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. To what temperature must the balloon be cooled to reduce its volume to 378 ml if the pressure. Chemistry gases combined gas law 1 answer tartar c. V is the volume of the gas.
Web catch the top stories of the day on anc’s ‘top story’ (20 july 2023) Web the combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. Web the most common form of the equation for the combined gas law is as follows: Pv = nrt where p = pressu. Combined gas law can be mathematically expressed as. Web the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three including the number of moles. Web if the number of moles of an ideal gas are kept constant under two different sets of conditions, a useful mathematical relationship called the combined gas law is. Web the combined gas law is a formula about ideal gases. T = temperature in kelvin. Web with the addition of avogadro's law, the combined gas law develops into the ideal gas law:
Combined Gas Law Practice 1 YouTube
For a combined gas law. 1.1 understanding the combined gas law 1.2 derivation of the combined gas law 2 solved example for you what is. Web this gas law is known as the combined gas law, and its mathematical form is. V is the volume of the gas. Chemistry gases combined gas law 1 answer tartar c.
Which equation represents the combined gas law?
Web this gas law is known as the combined gas law, and its mathematical form is. What is the proper form of the combined gas law? The ideal gas equation is : Web with the addition of avogadro's law, the combined gas law develops into the ideal gas law: Web catch the top stories of the day on anc’s ‘top.
Combined gas law Top 7 Facts YouTube
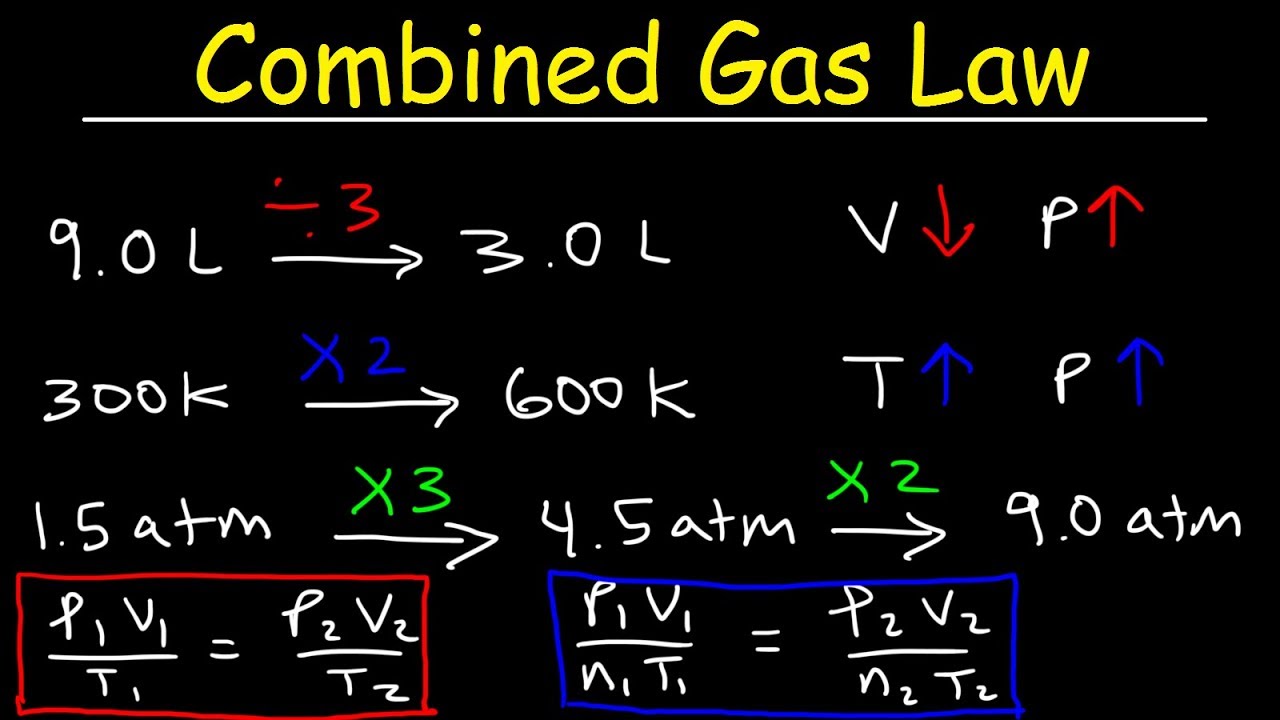
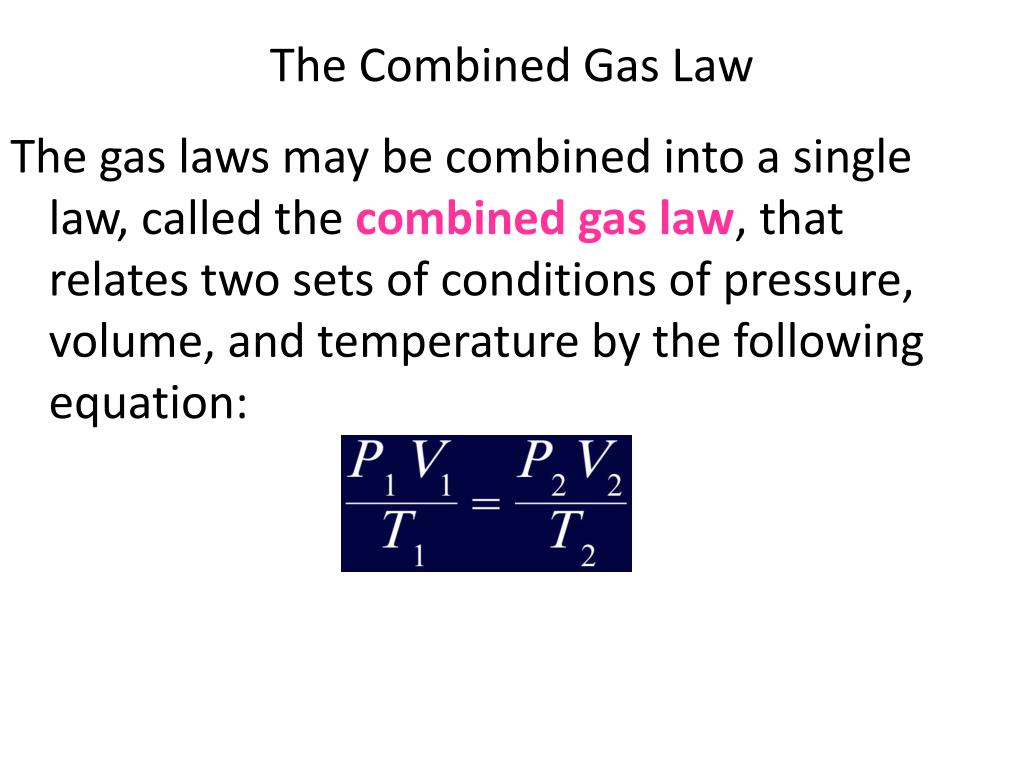
Combined gas law can be mathematically expressed as. This allows us to follow changes in all three. Web the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three including the number of moles. (1.5.1) p 1 v 1 t 1 = p 2 v 2 t 2 a t c o.
14.2 Combined Gas Law YouTube
(1.5.1) p 1 v 1 t 1 = p 2 v 2 t 2 a t c o n s t a n t n. Web with the addition of avogadro's law, the combined gas law develops into the ideal gas law: T is the temperature of the gas. Web the combined gas law relates the variables pressure, temperature, and.
PPT Gas Laws PowerPoint Presentation, free download ID5614600
To what temperature must the balloon be cooled to reduce its volume to 378 ml if the pressure. Web table of content 1 what is combined law formula? This allows us to follow changes in all three. Web what is the proper form of the combined gas law? K = constant (units of.
Gas Laws Presentation Chemistry
Web what is the proper form of the combined gas law? 1.1 understanding the combined gas law 1.2 derivation of the combined gas law 2 solved example for you what is. Apr 25, 2017 p v t = k (a constant). Web if the volume of a gas container at 32.0 c changes from 1.55 l to 755 ml, what.
PPT Combined Gas Law PowerPoint Presentation, free download ID3252378
To what temperature must the balloon be cooled to reduce its volume to 378 ml if the pressure. Web the combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas. V is the volume of the gas. Web combined gas law formula: Web the most common form of the equation for.
Gas Laws Ideal Gas Law Chemistry Net
Web table of content 1 what is combined law formula? Web combined gas law formula: Web with the addition of avogadro's law, the combined gas law develops into the ideal gas law: Web if the volume of a gas container at 32.0 c changes from 1.55 l to 755 ml, what will the final temperature be? T is the temperature.
Combined Gas Law Problems YouTube
To what temperature must the balloon be cooled to reduce its volume to 378 ml if the pressure. Chemistry gases combined gas law 1 answer tartar c. Web the most common form of the equation for the combined gas law is as follows: P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n.
PPT Gas Laws PowerPoint Presentation, free download ID5614600
T = temperature in kelvin. Web if the number of moles of an ideal gas are kept constant under two different sets of conditions, a useful mathematical relationship called the combined gas law is. Web catch the top stories of the day on anc’s ‘top story’ (20 july 2023) Web the combined gas law is a formula about ideal gases..
Web Combined Gas Law Formula:
Web with the addition of avogadro's law, the combined gas law develops into the ideal gas law: Web if the volume of a gas container at 32.0 c changes from 1.55 l to 755 ml, what will the final temperature be? It comes from putting together three different laws about the pressure , volume , and temperature of the gas. Web the combined gas law is a formula about ideal gases.
T = Temperature In Kelvin.
What is the proper form of the combined gas law? Web combined gas law formula. Combined gas law can be mathematically expressed as. P v = n r t {\displaystyle pv=nrt} where p is the pressure, v is volume, n is the.
V Is The Volume Of The Gas.
Apr 25, 2017 p v t = k (a constant). In order to compute the changes in temperature, pressure or. Web the most common form of the equation for the combined gas law is as follows: Web the combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of gas.
Web Table Of Content 1 What Is Combined Law Formula?
This allows us to follow changes in all three. Web catch the top stories of the day on anc’s ‘top story’ (20 july 2023) To what temperature must the balloon be cooled to reduce its volume to 378 ml if the pressure. Web the combined gas law relates the variables pressure, temperature, and volume whereas the ideal gas law relates these three including the number of moles.




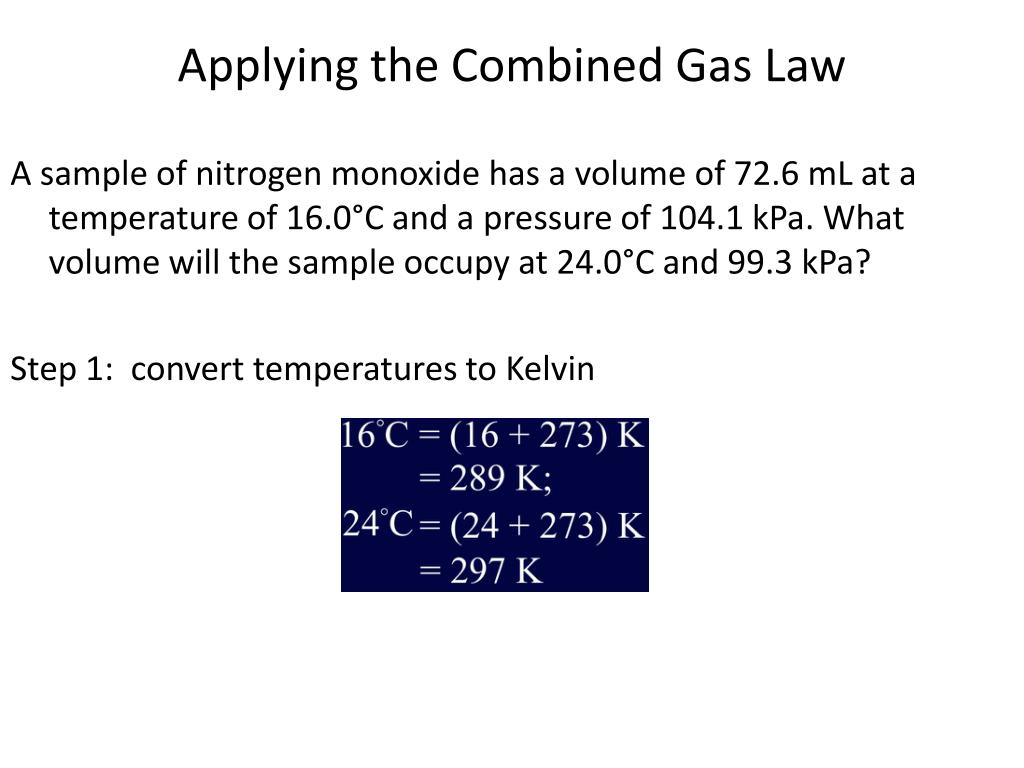
.PNG)