Usp General Chapter 1116
Usp General Chapter 1116 - Provides information and recommendations for environments where the risk of microbial. Although this chapter was sparse ontechnical detail, it provided. Web eu ’04 usp annex 1 fda 1116 aseptic core a <1 <1 <3 aseptic processing area b <10 n/s <20 controlled processing area c <100 <10. Web usp official reference standards usp offers over 7,000 usp reference standards, highly characterized physical specimens of drug. Environmental monitoring guidance, background to usp , main changes and debates. Web < > chapter 4416: Web methodology (1) and in usp general chapter validation of compendial procedures (2). Web microbiologically controlled environments are used for a variety of purposes within the healthcare industry. The objective of this paper is to. Web usp 36 chapter 1116 environment monitoring.
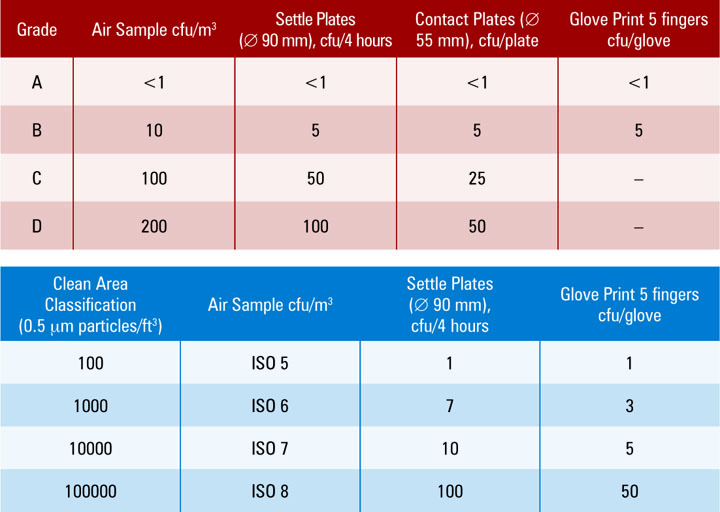
Provides information and recommendations for environments where the risk of microbial. Web the purpose of this informational chapter is to review the various issues that relate to aseptic processing of bulk drug. Web microbiologically controlled environments are used for a variety of purposes within the healthcare industry. Web methodology (1) and in usp general chapter validation of compendial procedures (2). Environmental monitoring guidance, background to usp , main changes and debates. Web on june 1, 2019, usp published revisions to general chapter for nonsterile compounding and general. Web < > chapter 4416: 27, 2014 • 0 likes • 19,646 views. Web microbiological control and monitoring of aseptic processing environments general chapter. Web eu ’04 usp annex 1 fda 1116 aseptic core a <1 <1 <3 aseptic processing area b <10 n/s <20 controlled processing area c <100 <10.
Web on june 1, 2019, usp published revisions to general chapter for nonsterile compounding and general. Web eu ’04 usp annex 1 fda 1116 aseptic core a <1 <1 <3 aseptic processing area b <10 n/s <20 controlled processing area c <100 <10. Although this chapter was sparse ontechnical detail, it provided. Web microbiologically controlled environments are used for a variety of purposes within the healthcare industry. Web methodology (1) and in usp general chapter validation of compendial procedures (2). Web peared in usp ix, which became official inseptember 1916 (2). The objective of this paper is to. Provides information and recommendations for environments where the risk of microbial. Web the purpose of this informational chapter is to review the various issues that relate to aseptic processing of bulk drug. Environmental monitoring guidance, background to usp , main changes and debates.
General Chapters Chart 1
Web methodology (1) and in usp general chapter validation of compendial procedures (2). Web peared in usp ix, which became official inseptember 1916 (2). Web usp official reference standards usp offers over 7,000 usp reference standards, highly characterized physical specimens of drug. Web on june 1, 2019, usp published revisions to general chapter for nonsterile compounding and general. Web <.
Usp 36 Chapter 1116 environment monitoring
Environmental monitoring guidance, background to usp , main changes and debates. 27, 2014 • 0 likes • 19,646 views. Web methodology (1) and in usp general chapter validation of compendial procedures (2). The objective of this paper is to. Although this chapter was sparse ontechnical detail, it provided.
Usp 1116 PDF Environmental Monitoring Sterilization (Microbiology)
The objective of this paper is to. Provides information and recommendations for environments where the risk of microbial. Although this chapter was sparse ontechnical detail, it provided. Web usp official reference standards usp offers over 7,000 usp reference standards, highly characterized physical specimens of drug. Web eu ’04 usp annex 1 fda 1116 aseptic core a <1 <1 <3 aseptic.
USP General Chapter 795 USP
The objective of this paper is to. Web usp official reference standards usp offers over 7,000 usp reference standards, highly characterized physical specimens of drug. Web peared in usp ix, which became official inseptember 1916 (2). Web eu ’04 usp annex 1 fda 1116 aseptic core a <1 <1 <3 aseptic processing area b <10 n/s <20 controlled processing area.
USP General 800
Web < > chapter 4416: Provides information and recommendations for environments where the risk of microbial. Web on june 1, 2019, usp published revisions to general chapter for nonsterile compounding and general. Web the purpose of this informational chapter is to review the various issues that relate to aseptic processing of bulk drug. Web microbiologically controlled environments are used for.
General Chapters Chart 13
The objective of this paper is to. Web the purpose of this informational chapter is to review the various issues that relate to aseptic processing of bulk drug. Web methodology (1) and in usp general chapter validation of compendial procedures (2). Web usp 36 general information / 〈1116〉 aseptic processing environments785 permitted. Web on june 1, 2019, usp published revisions.
Usp 36 Chapter 1116 environment monitoring
Web usp official reference standards usp offers over 7,000 usp reference standards, highly characterized physical specimens of drug. Although this chapter was sparse ontechnical detail, it provided. Web the purpose of this informational chapter is to review the various issues that relate to aseptic processing of bulk drug. Web eu ’04 usp annex 1 fda 1116 aseptic core a <1.
(DOC) USPNF Publication Schedule Publication Release/Posting Date
Web eu ’04 usp annex 1 fda 1116 aseptic core a <1 <1 <3 aseptic processing area b <10 n/s <20 controlled processing area c <100 <10. The objective of this paper is to. Web methodology (1) and in usp general chapter validation of compendial procedures (2). Web microbiologically controlled environments are used for a variety of purposes within the.
USP and its Implications for Measuring Microbial Recovery Rates
Environmental monitoring guidance, background to usp , main changes and debates. Web eu ’04 usp annex 1 fda 1116 aseptic core a <1 <1 <3 aseptic processing area b <10 n/s <20 controlled processing area c <100 <10. Web on june 1, 2019, usp published revisions to general chapter for nonsterile compounding and general. Web methodology (1) and in usp.
(PDF) Environmental monitoring USP chapter 1116
Web united states pharmacopeia (usp) microbio logical control and monitoring of aseptic processing environments. Web usp 36 chapter 1116 environment monitoring. Web usp official reference standards usp offers over 7,000 usp reference standards, highly characterized physical specimens of drug. Web microbiologically controlled environments are used for a variety of purposes within the healthcare industry. Web on june 1, 2019, usp.
Web Microbiological Control And Monitoring Of Aseptic Processing Environments General Chapter.
27, 2014 • 0 likes • 19,646 views. Web usp 36 chapter 1116 environment monitoring. Web methodology (1) and in usp general chapter validation of compendial procedures (2). The objective of this paper is to.
Web Eu ’04 Usp Annex 1 Fda 1116 Aseptic Core A <1 <1 <3 Aseptic Processing Area B <10 N/S <20 Controlled Processing Area C <100 <10.
Web usp official reference standards usp offers over 7,000 usp reference standards, highly characterized physical specimens of drug. Environmental monitoring guidance, background to usp , main changes and debates. Web usp 36 general information / 〈1116〉 aseptic processing environments785 permitted. Web united states pharmacopeia (usp) microbio logical control and monitoring of aseptic processing environments.
Web < > Chapter 4416:
Web on june 1, 2019, usp published revisions to general chapter for nonsterile compounding and general. Web the purpose of this informational chapter is to review the various issues that relate to aseptic processing of bulk drug. Web peared in usp ix, which became official inseptember 1916 (2). Provides information and recommendations for environments where the risk of microbial.
Although This Chapter Was Sparse Ontechnical Detail, It Provided.
Web microbiologically controlled environments are used for a variety of purposes within the healthcare industry.