Magnesium Reacts With Oxygen To Form
Magnesium Reacts With Oxygen To Form - Magnesium is in group 2 of the periodic table. Web magnesium reacts explosively with oxygen to form magnesium oxide. Which is not true of this reaction? We have been given that mg reacts with oxygen in the fixed ratio of 3 : Web magnesium is a group ii metal, and therefore has two electrons in it's highest energy level (or outermost electron shell). Web maybe we want to try to imagine the chemical system, which could look like this: Web magnesium metal reacts with the oxygen (o2) of the air to form magnesium oxide. Magnesium burns so bright because the reaction releases a lot of heat. Web answer (1 of 4): When the reaction with oxygen occurs, these two electrons are donated by the magnesium, forming positively charged mg 2+ ions.
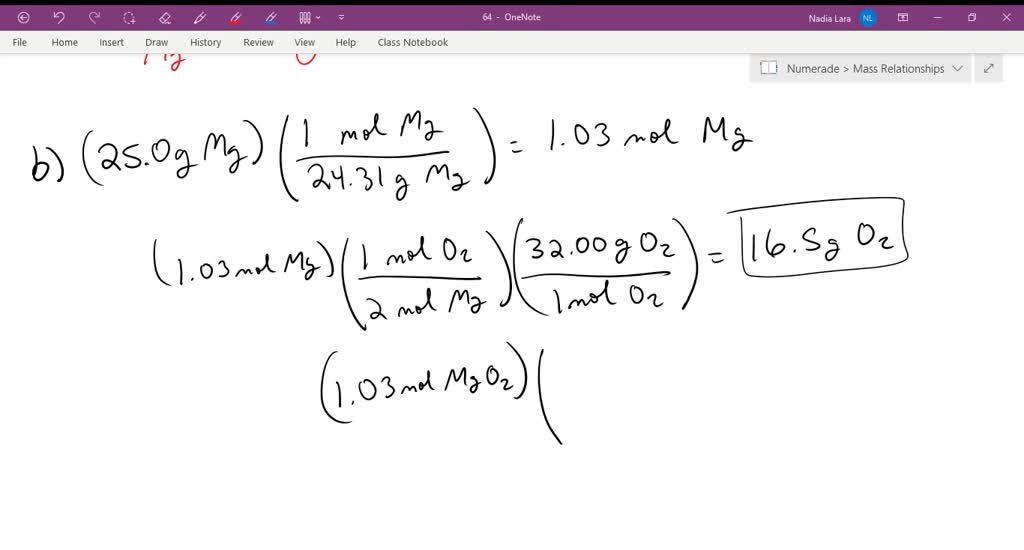
Magnesium is in group 2 of the periodic table. How many moles of oxygen molecules are needed to produce 10.0 moles of magnesium oxide ? Magnesium + oxygen magnesium oxide. So, 24g of mg requires =. Web potassium + oxygen potassium oxide. Thus, magnesium reacts with oxygen to form magnesium oxide. If 10.57 g of magnesium reacts completely with 6.96 g of oxygen, what is the percent by mass of oxygen in magnesium oxide?. Web magnesium has a charge of 2+, while oxygen has a charge of 2−. Web magnesium reacts with oxygen to form magnesium oxide, a) write a balanced chemical equation for this reaction. What evidence can you cite to back up your assertion that a chemical.
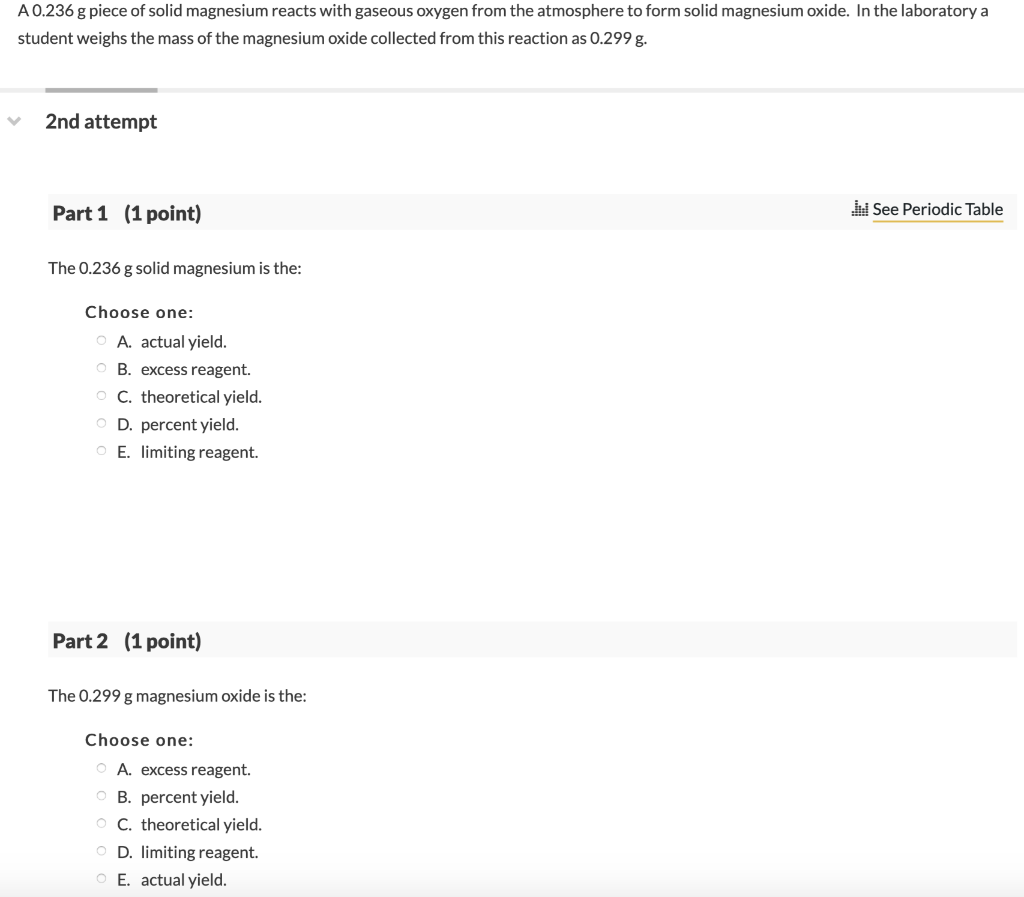
Web answer (1 of 4): How many grams are needed to obtain 1.5 mole of magnesium carbonate? Oxygen is in group 6 of the periodic table. 2mg + o 2 → 2mgo plus heat and light mistake points when the magnesium reacts with the oxygen. Web magnesium metal reacts with the oxygen (o2) of the air to form magnesium oxide. The reaction describes the formation of a new substance. Oxygen is a very elctronegative element, on the other hand magnesium is very electropositive. What evidence can you cite to back up your assertion that a chemical. Ncert 10th 1st chapter, experiment 1.1the video demonstrates how magnesium burns in air to form a white powder called magnesium. Oxygen is a diatomic molecule and is almost always reacting with elements as a gas, o2.
magnesium reacts with oxygen to form magnesium oxide equation
The reaction products are clear afterwards, and it adds a lot of light. The product of the reaction, magnesium oxide, is a. Web when magnesium reacts with oxygen, it produces light bright enough to blind you temporarily. Mg + o2 ® magnesium oxide. The burning of magnesium metal reacts with oxygen found in the air (oxygen gas) to form magnesium.
Simple Composition Reaction of magnesium and oxygen YouTube
A magnesium atom will lose 2 electrons to form a stable 2 + ion. Web answer (1 of 4): Just because you are heating something to get it to react does not mean there is a net flow of heat from the surrounding into the reaction. Web the reaction between magnesium and oxygen. The reaction products are clear afterwards, and.
Write the chemical equation describing the complete
Web magnesium reacts with oxygen. Web magnesium reacts with oxygen to form magnesium oxide, mgo. How many moles of oxygen molecules are needed to produce 10.0 moles of magnesium oxide? Web magnesium reacts explosively with oxygen to form magnesium oxide. Web magnesium’s reaction with oxygen is an interesting oxidation/reduction reaction because it shows the burning of a metal.
Reaction of Magnesium with Oxygen Gas (Burning Magnesium in Air) YouTube
How many moles of oxygen molecules are needed to produce 10.0 moles of magnesium oxide? Mg + o2 ® magnesium oxide. So, 24g of mg requires, 16g of oxygen to combine. Web magnesium is a group ii metal, and therefore has two electrons in it's highest energy level (or outermost electron shell). How many moles of oxygen molecules are needed.
"OMG Oxygen Magnesium" Poster by ivperisic Redbubble
Web in your case, magnesium metal and oxygen gas are the reactants and magnesium oxide is the product. How many moles are in 80.5g of aluminum sulfide? 2m g(s)+o2(g) → 2m go. Thus, magnesium reacts with oxygen to form magnesium oxide. Web maybe we want to try to imagine the chemical system, which could look like this:
Magnesium ribbon reacts with oxygen gas to form magnesium oxide. YouTube
The product of the reaction, magnesium oxide, is a. Web magnesium reacts with oxygen to form magnesium oxide, a) write a balanced chemical equation for this reaction. #2mg(s) + o_2(g) => 2mgo(s)# remember that combustion reactions occur when a species reacts with #o_2#, oxygen gas to release heat and produce a flame. Web magnesium metal reacts with the oxygen (o2).
Reaction of Magnesium and Oxygen YouTube
Web magnesium is a group ii metal, and therefore has two electrons in it's highest energy level (or outermost electron shell). The reaction products are clear afterwards, and it adds a lot of light. Web in your case, magnesium metal and oxygen gas are the reactants and magnesium oxide is the product. 3g of mg requires = 2g of oxygen..
SOLVEDMagnesium metal burns in oxygen to form magnesium oxide, MgO. (a
Web magnesium reacts with oxygen to form magnesium oxide, a) write a balanced chemical equation for this reaction. From these two masses, you calculate the percentage composition of magnesium oxide. If 10.57 g of magnesium reacts completely with 6.96 g of oxygen, what is the percent by mass of oxygen in magnesium oxide?. Oxygen is in group 6 of the.
Magnesium Oxide Uses, Benefits, Dosage, Side Effects
How many moles of oxygen molecules are needed to produce 10.0 moles of magnesium oxide ? How many moles of oxygen molecules are needed to produce 10.0 moles of magnesium oxide? A short video that shows magnesium reacting with oxygen. 2mg + o 2 → 2mgo plus heat and light mistake points when the magnesium reacts with the oxygen. Web.
Magnesium reacts with hydrochloric acid Stock Image C028/0732
If 10.57 g of magnesium reacts completely with 6.96 g of oxygen, what is the percent by mass of oxygen in magnesium oxide?. So, 24g of mg requires, 16g of oxygen to combine. After it burns, it forms a white powder of the magnesium oxide. Mg + o2 = mgo b) what is the ratio of the moles of magnesium.
Magnesium Gives Up Two Electrons To Oxygen Atoms To Form This Powdery Product.
Step by step solved in 3 steps see solution check out a sample q&a here knowledge booster learn. Web if magnesium reacts with oxygen to produce magnesium oxide only on the application of heat, then why isn't it categorised as an endothermic reaction? The mass of magnesium oxide produced equals the mass of magnesium consumed plus the mass of oxygen consumed. 2mg(s) + o 2 (g) → 2mgo(s)
If 10.57 G Of Magnesium Reacts Completely With 6.96 G Of Oxygen, What Is The Percent By Mass Of Oxygen In Magnesium Oxide?.
4k(s) + o 2 (g) 2k 2 o(s) magnesium reacts readily in air burning with a white light. 304k views 12 years ago. Web potassium + oxygen potassium oxide. 2mg(s) + o2(g) → 2mgo(s) you know that 10.0 g of magnesium take part in this reaction and that 16.6 g of magnesium oxide are produced.
Oxygen Is A Very Elctronegative Element, On The Other Hand Magnesium Is Very Electropositive.
Web magnesium has a charge of 2+, while oxygen has a charge of 2−. Magnesium reacts with oxygen to create magnesium oxide. The burning of magnesium metal reacts with oxygen found in the air (oxygen gas) to form magnesium oxide. How many moles of oxygen molecules are needed to produce 10.0 moles of magnesium oxide ?
The Two Electrons Donated From One Magnesium Atom Are Taken Up By An Oxygen Atom.
Which is not true of this reaction? Web magnesium reacts with oxygen to form magnesium oxide, mgo. Magnesium combines with oxygen to form magnesium oxide. So, 24g of mg requires, 16g of oxygen to combine.