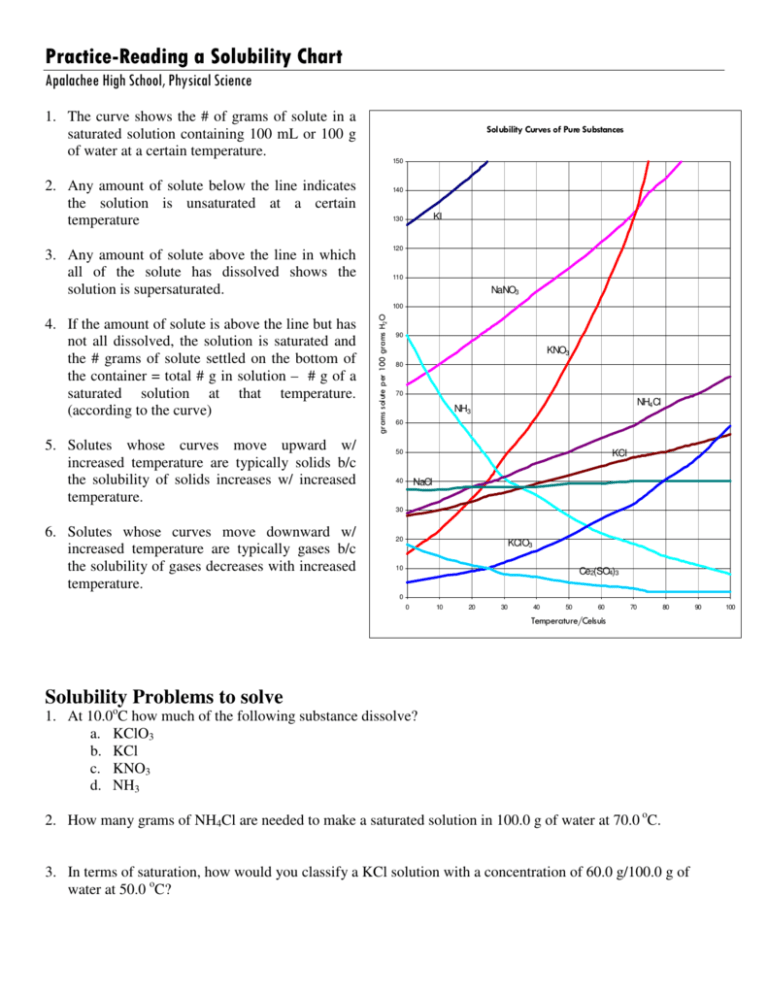
How To Read Solubility Curves
How To Read Solubility Curves - 2) the ph of the solution at equivalence point is dependent. The maximum amount of a substance that will dissolve in a certain amount of solvent at a given temperature is called that substances solubility in that solvent. Web learn if a solution is saturated or unsaturated by reading a solubility curve. What is the likely identity of the unknown?. Web the variation in the solubility of any given substance with the change of temperature is shown by the solubility curve. Both in g/100 ml g / 100 m l of water. Web 0:00 / 15:10 solubility curves explained chem academy 33.7k subscribers subscribe 838 94k views 8 years ago solutions in this video i will explain solubility curves. Solubility curves for more than one substance are often drawn on the same. I assume both cross solubilities are related to the volume of water used for the initial solution, not of the initial solution volume, i.e. At 40ºc, exactly 64 g of an unknown salt dissolved in 100 g of water.
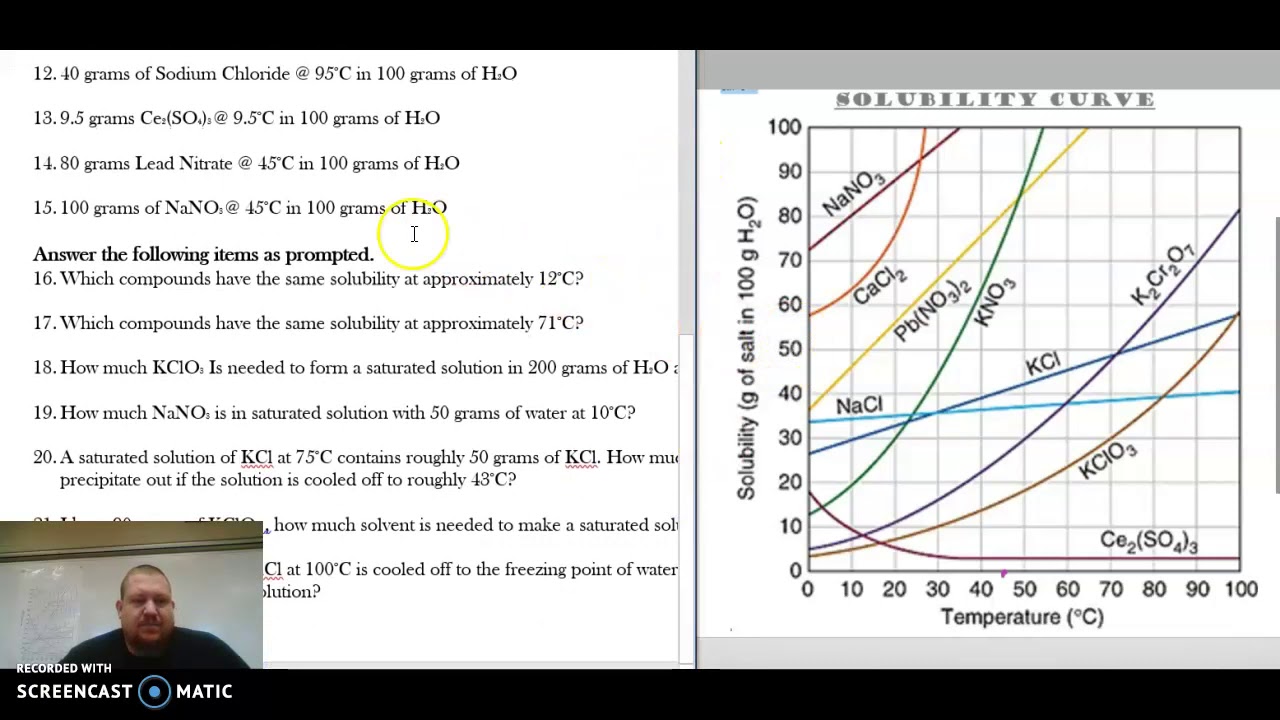
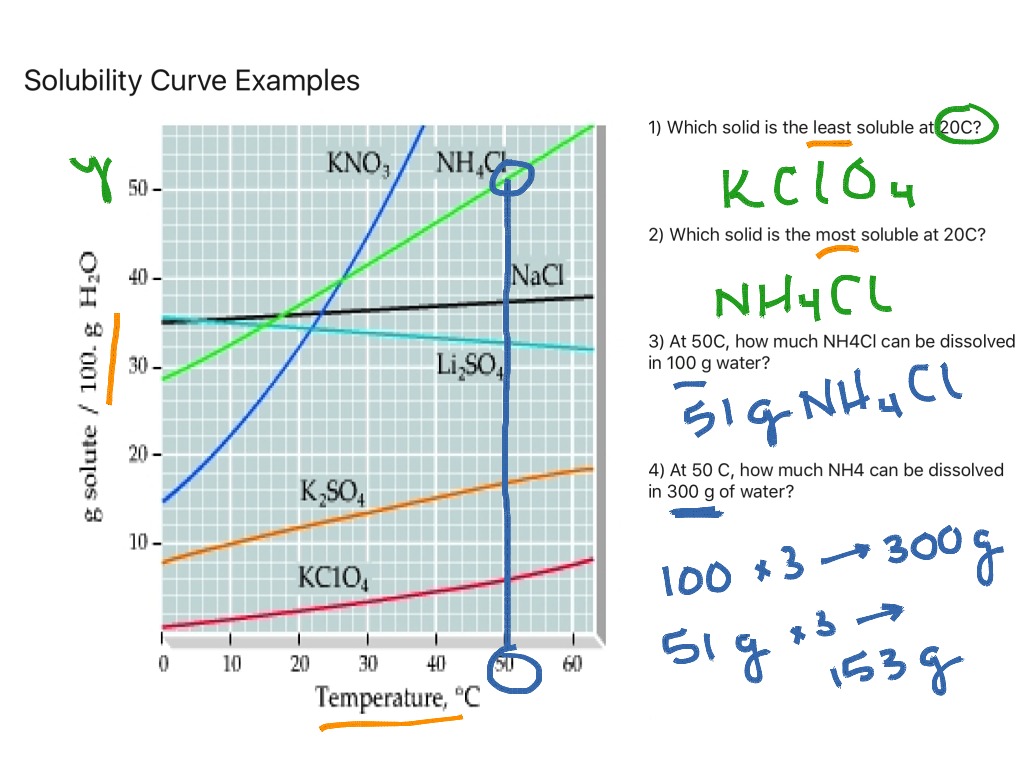
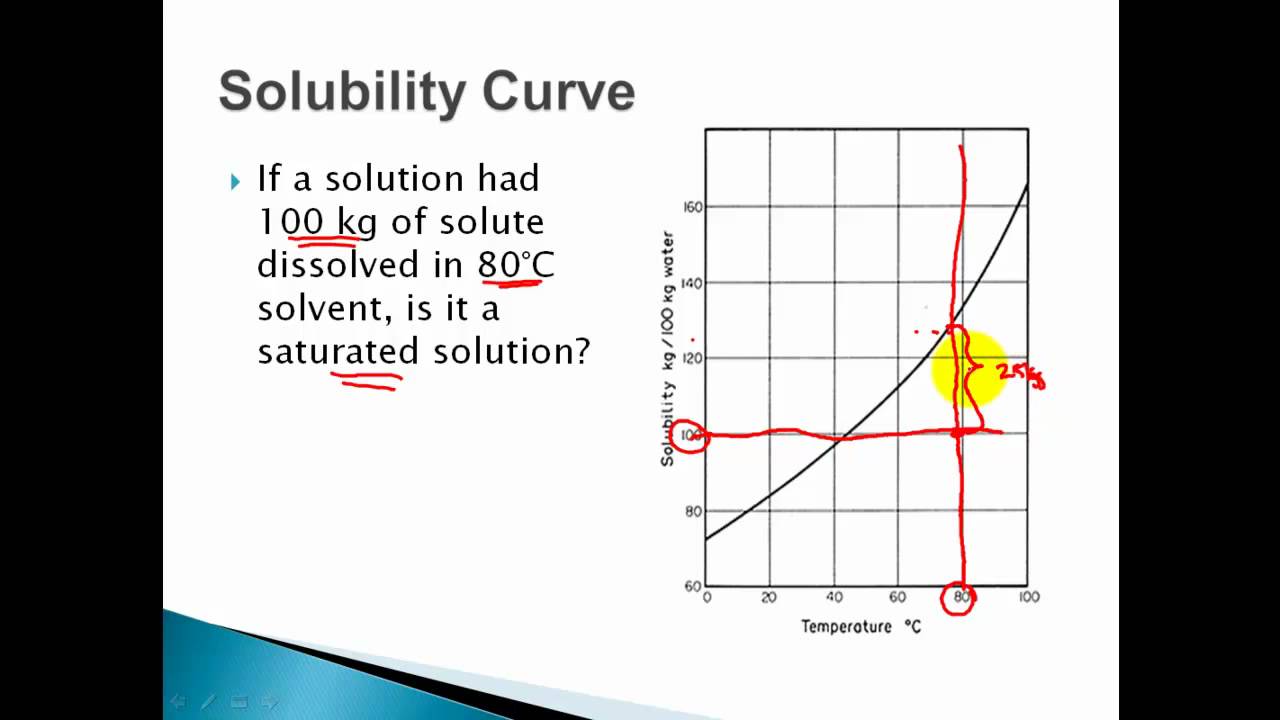
Web how to read a solubility graph. Web 0:00 / 15:10 solubility curves explained chem academy 33.7k subscribers subscribe 838 94k views 8 years ago solutions in this video i will explain solubility curves. Web the graph will typically have a curve that shows the solubility as a function of temperature. 2) the ph of the solution at equivalence point is dependent. From reading a solubility graph, one can determine the mass of solute that can dissolve at specific temperatures,. A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. 68k views 7 years ago. The maximum amount of a substance that will dissolve in a certain amount of solvent at a given temperature is called that substances solubility in that solvent. Web how to read a solubility curve 1. At 40ºc, exactly 64 g of an unknown salt dissolved in 100 g of water.
How much kclwould be able to dissolve in 100 g of water at 50ºc? Practice reading a solubility graph—part 2. The curve line drawn on a graph showing the relationship between temperature and solubility of the substance. 68k views 7 years ago. The maximum amount of a substance that will dissolve in a certain amount of solvent at a given temperature is called that substances solubility in that solvent. Solubility curves for more than one substance are often drawn on the same. At 40ºc, exactly 64 g of an unknown salt dissolved in 100 g of water. Practice reading a solubility graph—part 1. Web this chemistry video tutorial provides a basic introduction into solubility curves. I assume both cross solubilities are related to the volume of water used for the initial solution, not of the initial solution volume, i.e.
Read Solubility Curve Practice Answers / Hw Solubility Curve Worksheet
Web 0:00 / 15:10 solubility curves explained chem academy 33.7k subscribers subscribe 838 94k views 8 years ago solutions in this video i will explain solubility curves. Practice reading a solubility graph—part 2. A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Web how to read a solubility graph. The maximum amount.
PracticeReading a Solubility Chart
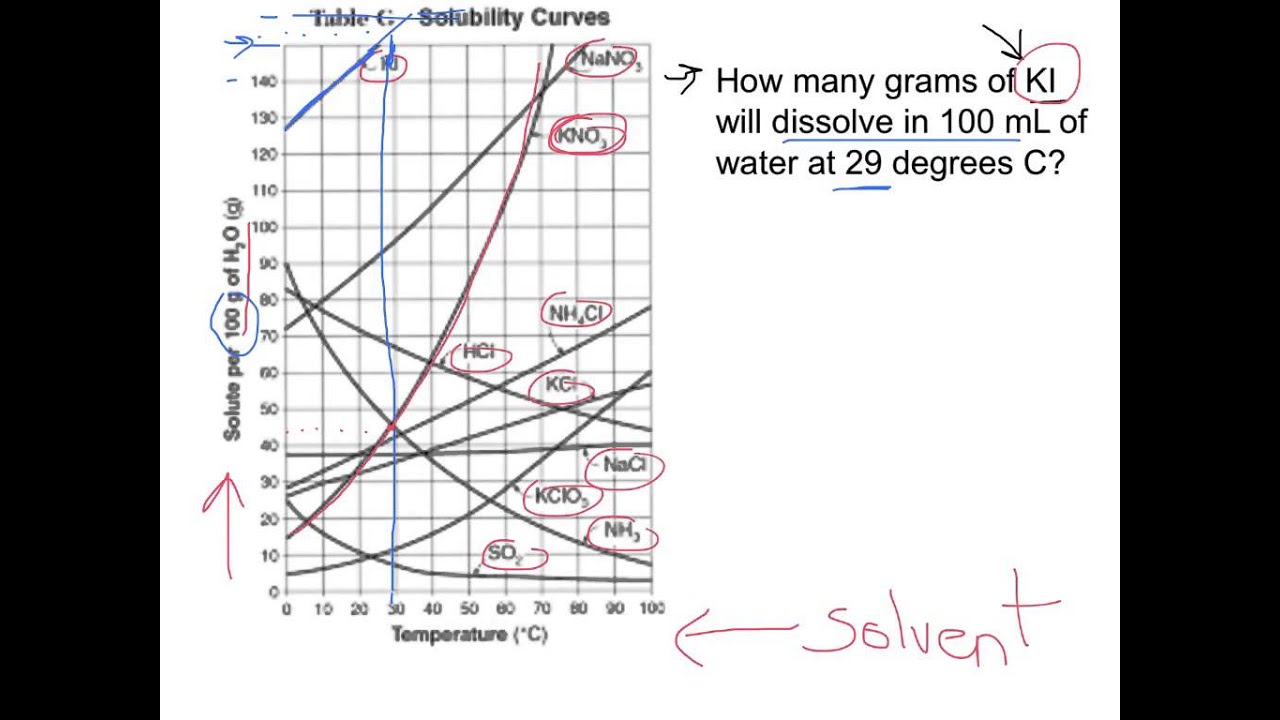
To read the graph, find the line for the substance. Example questions what mass of solute will dissolve in 100g of water at the following temperatures? The curve line drawn on a graph showing the relationship between temperature and solubility of the substance. Web how to read a solubility graph. Both in g/100 ml g / 100 m l of.
PPT Solubility curve PowerPoint Presentation, free download ID6497715
Solubility curves for more than one substance are often drawn on the same. Practice reading a solubility graph—part 1. Web this chemistry video tutorial provides a basic introduction into solubility curves. The solubility curve is a graph that shows how the concentration of a solute. I assume both cross solubilities are related to the volume of water used for the.
Read Solubility Curve Practice Answers / PPT UNIT 1C Reading
Web learn if a solution is saturated or unsaturated by reading a solubility curve. 68k views 7 years ago. How to read the solubility curve? Web solubility curvesare used to show how the solubility of a substance changes with temperature. Web the graph will typically have a curve that shows the solubility as a function of temperature.
Interpreting Solubility Curves YouTube
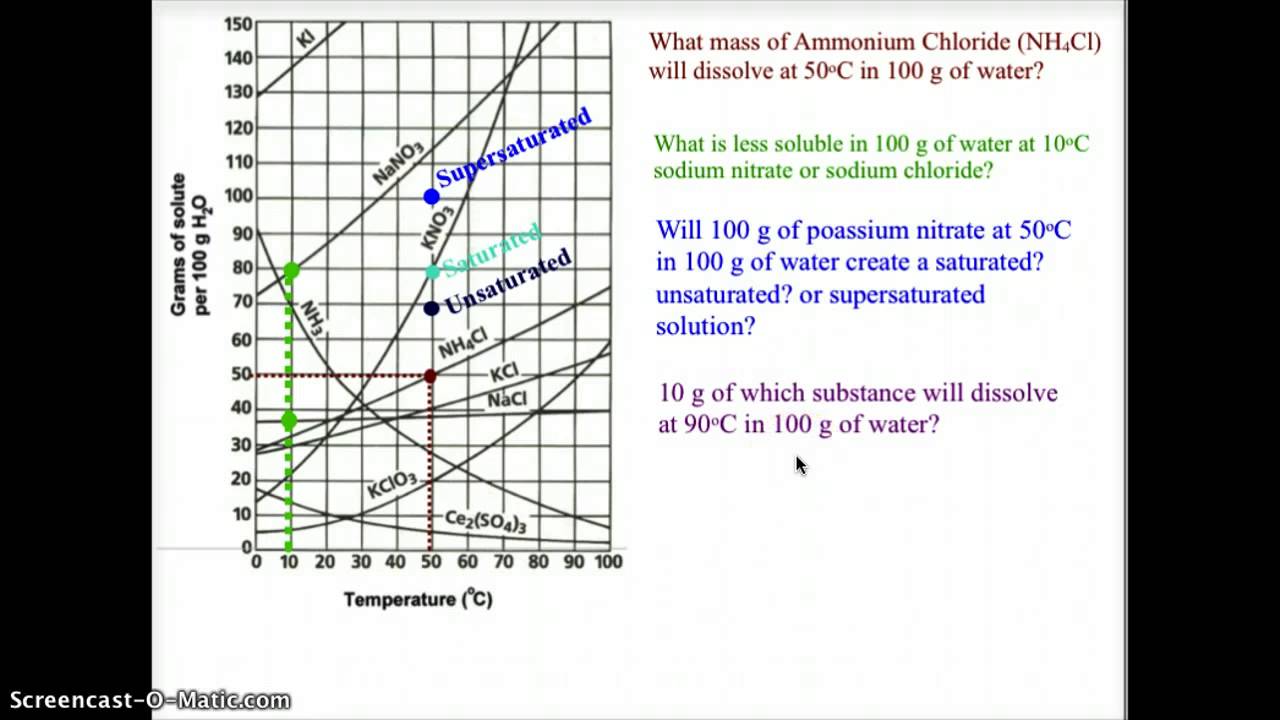
How much kclwould be able to dissolve in 100 g of water at 50ºc? 68k views 7 years ago. 2) the ph of the solution at equivalence point is dependent. What is the likely identity of the unknown?. A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c.
Reading solubility curves YouTube
Web learn if a solution is saturated or unsaturated by reading a solubility curve. 68k views 7 years ago. Web a solubility graph is drawn to display the solubility at different temperatures. It contains plenty of examples and practice problems on calculating the solubility of an ionic compound at a. I assume both cross solubilities are related to the volume.
Practice Reading Solubility Curve Pt. 1 YouTube
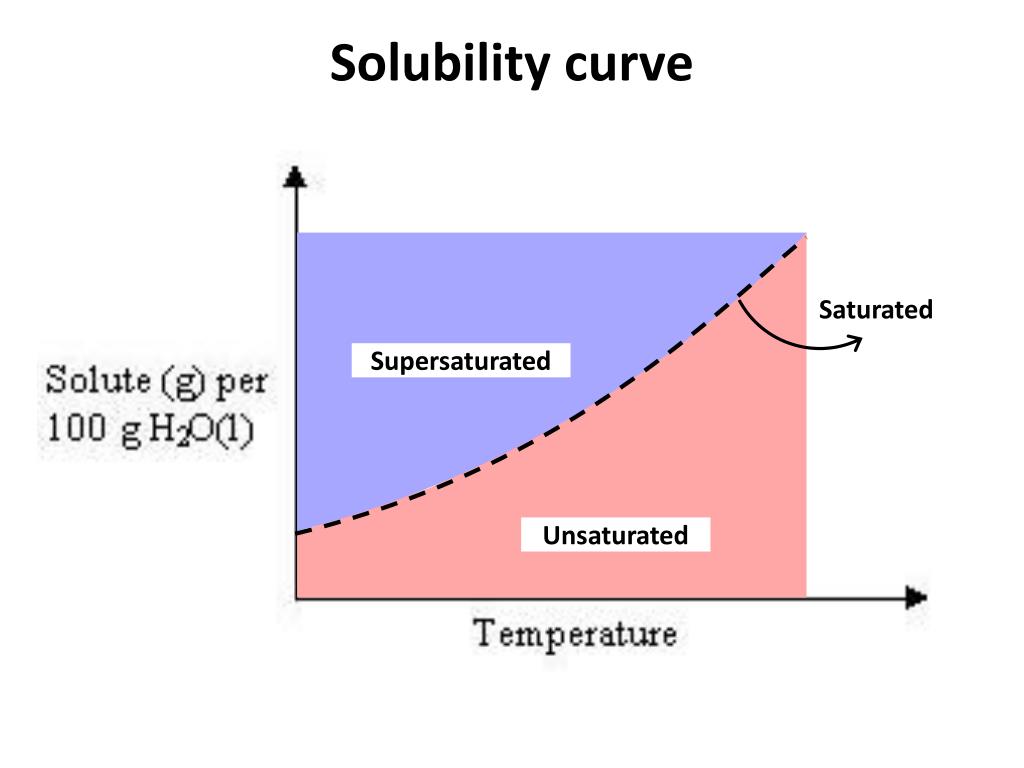
Web a solubility graph is drawn to display the solubility at different temperatures. The maximum amount of a substance that will dissolve in a certain amount of solvent at a given temperature is called that substances solubility in that solvent. Web learn if a solution is saturated or unsaturated by reading a solubility curve. Web how to read a solubility.
Solubility & Solubility Curves Science, Chemistry ShowMe
The solubility curve is a graph that shows how the concentration of a solute. Web this chemistry video tutorial provides a basic introduction into solubility curves. How much kclwould be able to dissolve in 100 g of water at 50ºc? Web 0:00 / 15:10 solubility curves explained chem academy 33.7k subscribers subscribe 838 94k views 8 years ago solutions in.
Solubility Curves Saturated, Unsaturated, Supersaturated Solutions
Web 0:00 / 15:10 solubility curves explained chem academy 33.7k subscribers subscribe 838 94k views 8 years ago solutions in this video i will explain solubility curves. Web how to read a solubility graph. A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. 68k views 7 years ago. 2) the ph of.
Reading a SolubilityChart.doc Google Docs
From reading a solubility graph, one can determine the mass of solute that can dissolve at specific temperatures,. Example questions what mass of solute will dissolve in 100g of water at the following temperatures? How to read the solubility curve? To read the graph, find the line for the substance. Web how to read a solubility curve 1.
The Solubility Curve Is A Graph That Shows How The Concentration Of A Solute.
How to read the solubility curve? Example questions what mass of solute will dissolve in 100g of water at the following temperatures? Web reading solubility curves what is solubility? How much kclwould be able to dissolve in 100 g of water at 50ºc?
Practice Reading A Solubility Graph—Part 2.
Solubility curves for more than one substance are often drawn on the same. The maximum amount of a substance that will dissolve in a certain amount of solvent at a given temperature is called that substances solubility in that solvent. Web the graph will typically have a curve that shows the solubility as a function of temperature. Practice reading a solubility graph—part 1.
The Curve Line Drawn On A Graph Showing The Relationship Between Temperature And Solubility Of The Substance.
A solubility curve is a graph of solubility, measured in g/100 g water, against temperature in °c. Web learn if a solution is saturated or unsaturated by reading a solubility curve. Both in g/100 ml g / 100 m l of water. Web solubility curvesare used to show how the solubility of a substance changes with temperature.
Web 0:00 / 15:10 Solubility Curves Explained Chem Academy 33.7K Subscribers Subscribe 838 94K Views 8 Years Ago Solutions In This Video I Will Explain Solubility Curves.
I assume both cross solubilities are related to the volume of water used for the initial solution, not of the initial solution volume, i.e. From reading a solubility graph, one can determine the mass of solute that can dissolve at specific temperatures,. What is the likely identity of the unknown?. 2) the ph of the solution at equivalence point is dependent.