How Many Bonds Does Hydrogen Form
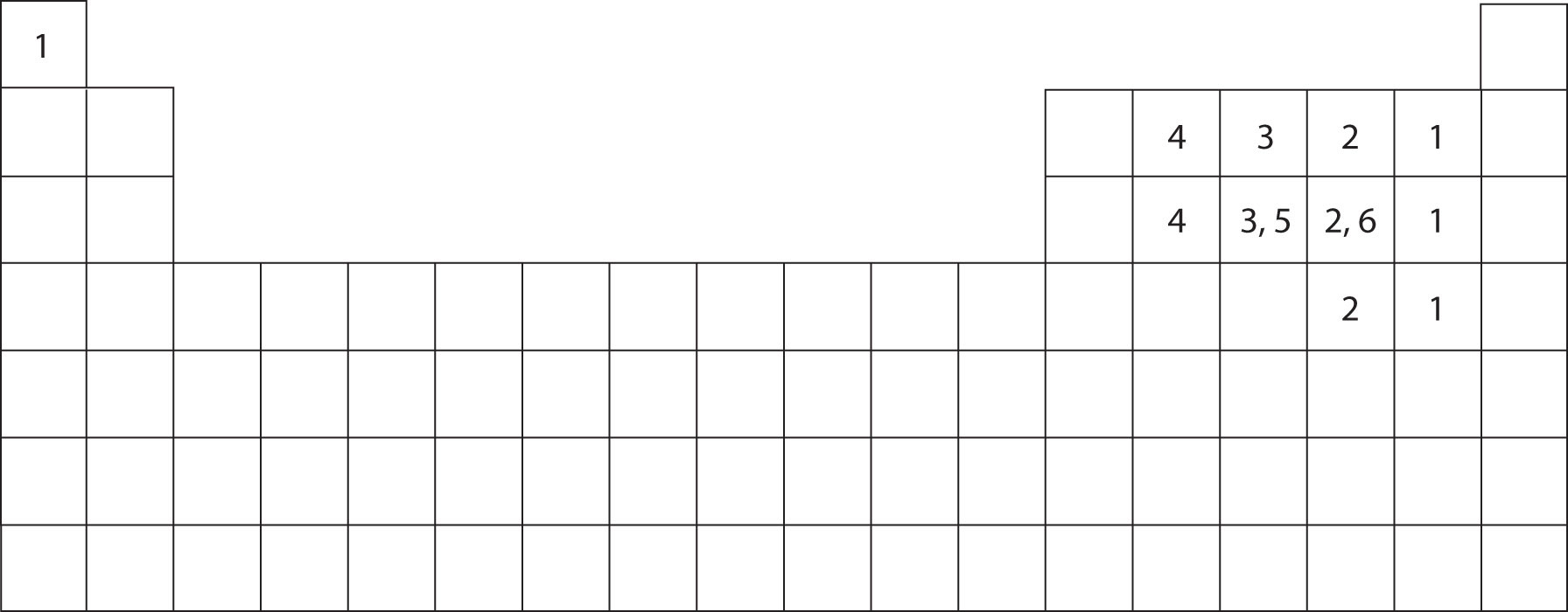
How Many Bonds Does Hydrogen Form - Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. In this question, we need to determine the. Web hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. Thus one hydrogen atom will only bond once. Using pauling's scale—c (2.55) and h. Web hydrogen is nonmetallic (except when it becomes metallic at extremely high pressures) and readily forms a single covalent bond with most nonmetallic elements, forming. Web nov 7, 2016. Two with the hydrogen atoms and two with the with. Since hydrogen has only one valence electron, it will only bond once.
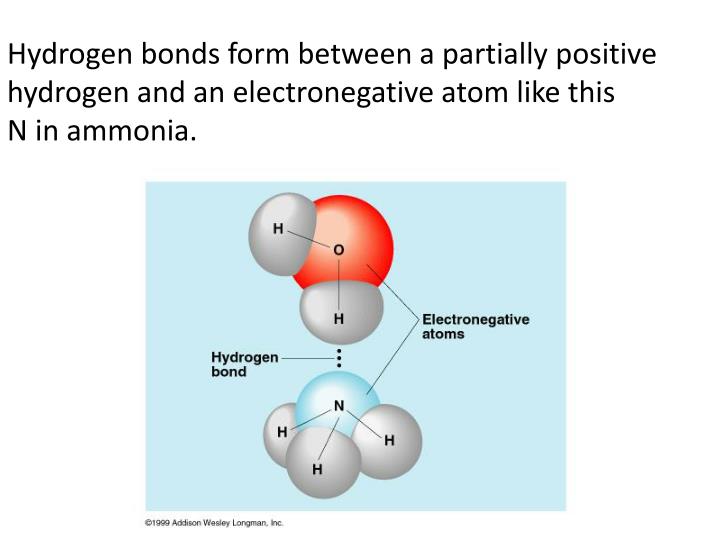
What is called the number of bonds an atom can. Two with the hydrogen atoms and two with the with. Hydrogen bonds are extremely important in biology, as they are the reason for the structure of dna and its properties. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. It's these hydrogen bonds that give water many of its properties. Using pauling's scale—c (2.55) and h. This leads to the hexagonal cage structure of crystalline ice. Such molecules will always have higher. Web hydrogen bonding in dna.
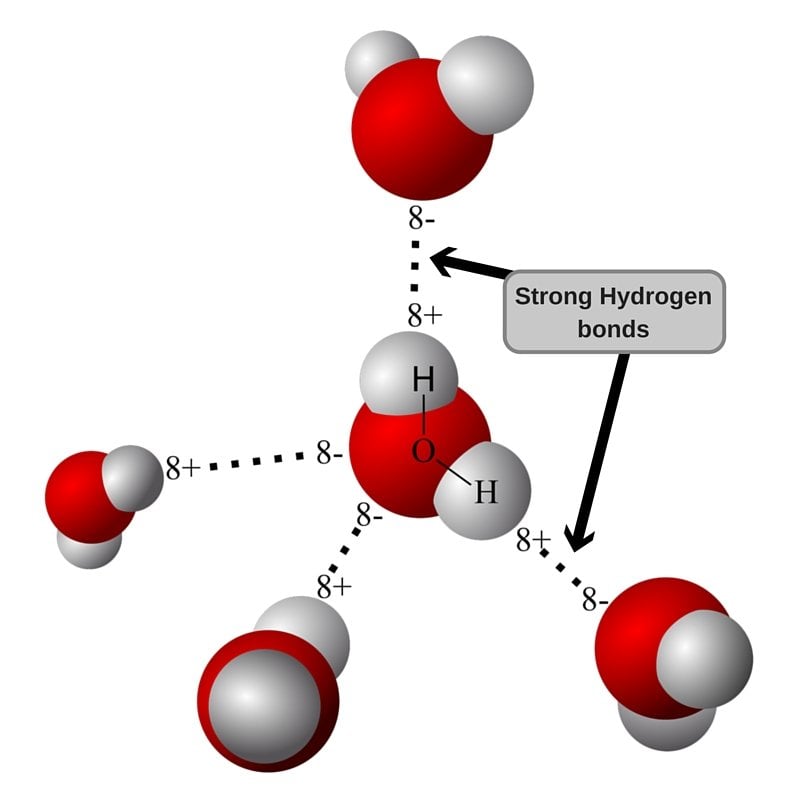
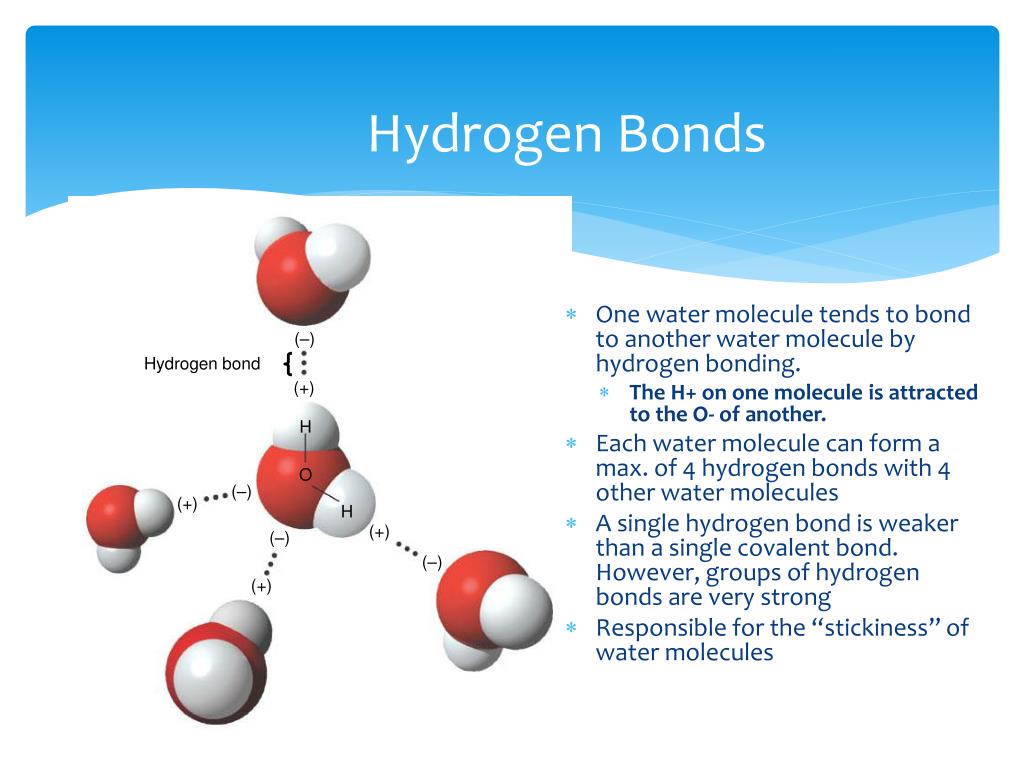
Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Web a hydrogen atom will typically form two covalent bonds because hydrogen has two electrons to share in a bond. Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. This leads to the hexagonal cage structure of crystalline ice. Web hydrogen forms weak bonds between molecules, latching onto adjacent oxygen, nitrogen or fluorine atoms. Intramolecular hydrogen bonding intermolecular hydrogen bonding now understand. Hydrogen bonds are extremely important in biology, as they are the reason for the structure of dna and its properties. In this question, we need to determine the. Web nov 7, 2016. Thus one hydrogen atom will only bond once.
Why Do Fingers/Hands Stick To Ice? » Science ABC
Using pauling's scale—c (2.55) and h. Web nov 7, 2016. Two with the hydrogen atoms and two with the with. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules:
Hydrogen bonds YouTube
Since hydrogen has only one valence electron, it will only bond once. Web there are two types of hydrogen bondings which are given below. What is called the number of bonds an atom can. Web nov 7, 2016. Web hydrogen is nonmetallic (except when it becomes metallic at extremely high pressures) and readily forms a single covalent bond with most.
PPT Chemistry—A Quick Review PowerPoint Presentation ID3103671
It's these hydrogen bonds that give water many of its properties. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Web hydrogen forms weak bonds between molecules, latching onto adjacent oxygen, nitrogen or fluorine atoms. This leads to the hexagonal cage structure of crystalline ice. Thus one hydrogen.
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Hydrogen bonds are extremely important in biology, as they are the reason for the structure of dna and its properties. Intramolecular hydrogen bonding intermolecular hydrogen bonding now understand. It's these hydrogen bonds that give water many of its properties..
2.2 Chemical Bonds Anatomy & Physiology
Hydrogen bonds are extremely important in biology, as they are the reason for the structure of dna and its properties. Thus one hydrogen atom will only bond once. It's these hydrogen bonds that give water many of its properties. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding..
PPT Properties of Water PowerPoint Presentation, free download ID
It's these hydrogen bonds that give water many of its properties. Using pauling's scale—c (2.55) and h. Thus one hydrogen atom will only bond once. Intramolecular hydrogen bonding intermolecular hydrogen bonding now understand. Such molecules will always have higher.
Bonds That Hold Water Molecules Together / Intermolecular Forces
Since hydrogen has only one valence electron, it will only bond once. Web hydrogen is nonmetallic (except when it becomes metallic at extremely high pressures) and readily forms a single covalent bond with most nonmetallic elements, forming. Web hydrogen forms weak bonds between molecules, latching onto adjacent oxygen, nitrogen or fluorine atoms. Thus one hydrogen atom will only bond once..
how many bonds does sulfur form
This leads to the hexagonal cage structure of crystalline ice. In this question, we need to determine the. Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. Web a hydrogen atom will typically form two covalent bonds because hydrogen has two electrons to share in a bond. It's these hydrogen bonds that give.
Properties of Water Presentation Biology
Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Such molecules will always have higher. What is called the number of bonds an atom can. Hydrogen bonds are extremely important in biology, as they are the reason for the structure of dna and its properties. Web hydrogen bonds.
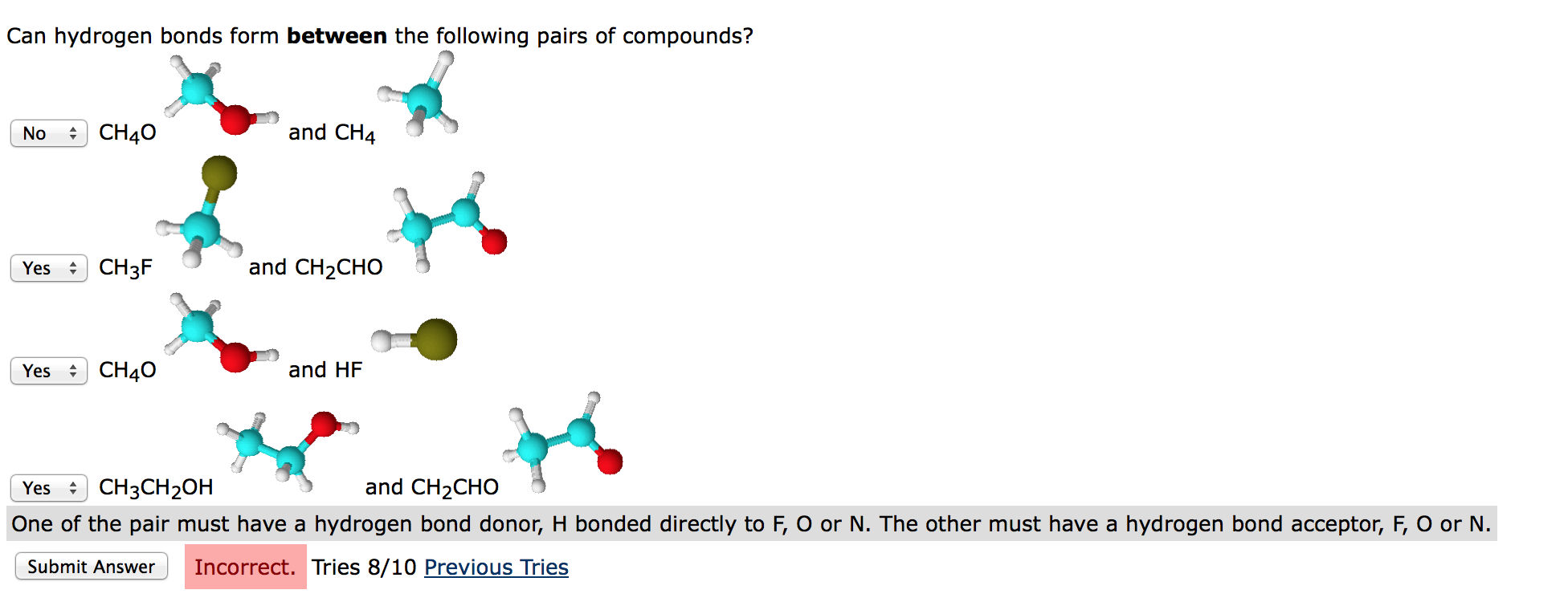
Solved Can hydrogen bonds form between the following pairs
Web there are two types of hydrogen bondings which are given below. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Since hydrogen has only one valence electron, it will only bond once. Web nov 7, 2016. Hydrogen bonds are extremely important in biology, as they are the.
Two With The Hydrogen Atoms And Two With The With.
It's these hydrogen bonds that give water many of its properties. Such molecules will always have higher. Web hydrogen bond strengths range from 4 kj to 50 kj per mole of hydrogen bonds. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules:
Thus One Hydrogen Atom Will Only Bond Once.
Web hydrogen bonding in dna. What is called the number of bonds an atom can. Since hydrogen has only one valence electron, it will only bond once. In this question, we need to determine the.
Web Hydrogen Forms Weak Bonds Between Molecules, Latching Onto Adjacent Oxygen, Nitrogen Or Fluorine Atoms.
Web hydrogen is nonmetallic (except when it becomes metallic at extremely high pressures) and readily forms a single covalent bond with most nonmetallic elements, forming. Web nov 7, 2016. Web there are two types of hydrogen bondings which are given below. Intramolecular hydrogen bonding intermolecular hydrogen bonding now understand.
Web Hydrogen Bonds Form Between The Hydrogen Atom Of One Water Molecule And The Lone Pair On The Oxygen Atom Of Another Molecule.
Web a hydrogen atom will typically form two covalent bonds because hydrogen has two electrons to share in a bond. Hydrogen bonds are extremely important in biology, as they are the reason for the structure of dna and its properties. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. This leads to the hexagonal cage structure of crystalline ice.




.PNG)