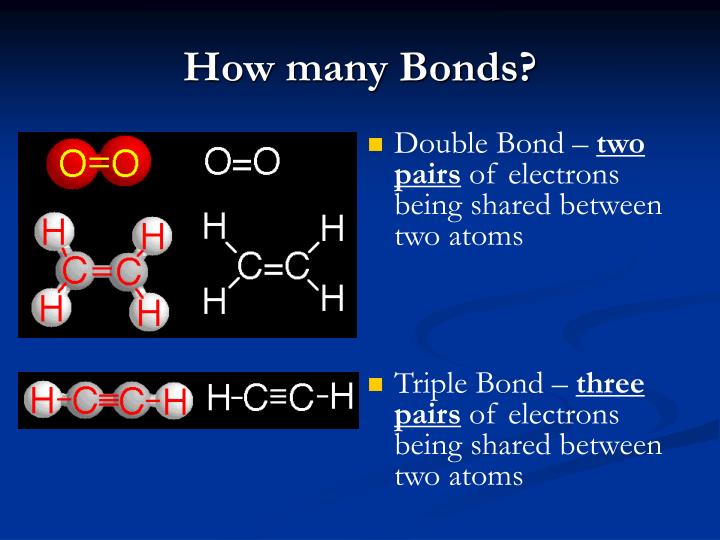
How Many Bonds Does Br Form
How Many Bonds Does Br Form - ___ 1, 4, 1, 2, 1, 3, 1 wedges and dashes. Group 5a form 3 bonds; Web how many rings does jupiter have; Typically, boron forms 3 covalent bonds. 2)how many bonds does br form in the compound kbr? A financial ratio that expresses the leverage of a bond issuer. Web 1) how many bonds does i form in the compound ki? Web as we know that bromine lies in the same group as chlorine and fluorine, bromine has 7 electrons in its valence shell. Basically 3 lone pairs on each br. For example, it can form two bonds with.
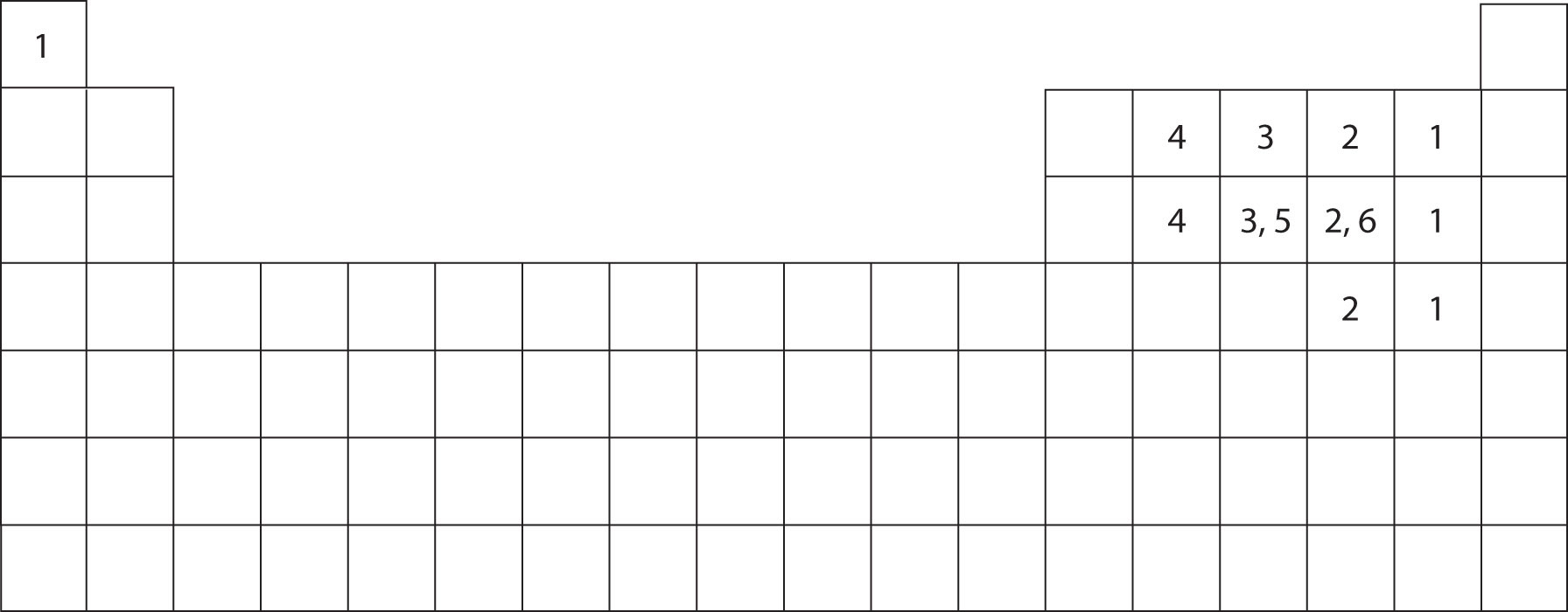
Members of a group typically have similar properties and electron configurations in their outer shell. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence. Web typically, the atoms of group 4a form 4 covalent bonds; 3) how many bonds does each s atom form? If they can only form. How many bonds does n form? A vertical column in the periodic table. Group 6a form 2 bonds; Modeling lonic and covalent bonds part 1: Web how many bonds does each element usually form with other atoms in a compound?
Typically, boron forms 3 covalent bonds. Web how many bonds does h form with other compounds? Web this document contains the past and current bond formulas for activity code 1 (importer/broker) continuous bonds. Group 5a form 3 bonds; How many bonds does br form? Web typically, the atoms of group 4a form 4 covalent bonds; Web 9) based on your answer to question 7, explain why h, f, cl and br are most likely to be end (terminal) atoms rather than central (bridging) atoms in a molecule. Web 1) how many bonds does i form in the compound ki? Group 6a form 2 bonds; If they can only form.
__TOP__ How Many Covalent Bonds Can Chlorine Form
How many bonds does n form? Boron can form a fourth covalent bond and thus acquire a formal negative charge. For example, it can form two bonds with. Web 9) based on your answer to question 7, explain why h, f, cl and br are most likely to be end (terminal) atoms rather than central (bridging) atoms in a molecule..
PPT What are bonds? PowerPoint Presentation, free download ID5980343
3) how many bonds does each s atom form? 0 how many bonds will a carbon atom form with four bromine atoms? Web how many rings does jupiter have; Basically 3 lone pairs on each br. Web 1) how many bonds does i form in the compound ki?
what type of bonds are shown in this diagram HanakaNao
Group 5a form 3 bonds; It can form 1, 2, or 3 bonds with other atoms. Web how many bonds does h form with other compounds? How many bonds does f form? Web this document contains the past and current bond formulas for activity code 1 (importer/broker) continuous bonds.
How Many Single Bonds Can Carbon Form fredhughesdesign
How many bonds does n form? 2)how many bonds does br form in the compound kbr? 3) how many bonds does each s atom form? Basically 3 lone pairs on each br. Web how many bonds does h form with other compounds?
PPT Chapter 5 Atoms & Bonding PowerPoint Presentation ID5979513
Web this document contains the past and current bond formulas for activity code 1 (importer/broker) continuous bonds. Boron can form a fourth covalent bond and thus acquire a formal negative charge. Typically, boron forms 3 covalent bonds. 2)how many bonds does br form in the compound kbr? Web how many bonds does each element usually form with other atoms in.
How Many Bonds Does Nitrogen Form tour bous
Typically, boron forms 3 covalent bonds. In the case of dibromine, the combined. And group 7a form one bond. Web how many bonds does each element usually form with other atoms in a compound? Build america bonds are taxable municipal bonds that carry special tax credits and federal subsidies for either the bond issuer or the bondholder.
HONC 1234 ChemSimplified
In the case of dibromine, the combined. Web how many bonds does h form with other compounds? Web bromine forms covalent bonds because it has seven valence electrons, but neon has eight valence electrons and already fulfills the octet rule. A financial ratio that expresses the leverage of a bond issuer. Members of a group typically have similar properties and.
how many bonds does sulfur form
And group 7a form one bond. Web 9) based on your answer to question 7, explain why h, f, cl and br are most likely to be end (terminal) atoms rather than central (bridging) atoms in a molecule. How many bonds does br form? Web how many bonds does h form with other compounds? How many bonds does n form?
What Are I Bonds & How Do They Work? Forbes Advisor
3) how many bonds does each s atom form? Web bromine is capable of forming one bond when it is in its elemental form. How many bonds does br form. •br br submit your choice. How many bonds does f form?
Solved Name Course/section Instructor Name Date PreLab
Web 1) how many bonds does i form in the compound ki? Build america bonds are taxable municipal bonds that carry special tax credits and federal subsidies for either the bond issuer or the bondholder. For example, it can form two bonds with. Group 5a form 3 bonds; Group 6a form 2 bonds;
Web How Many Bonds Does Each Element Usually Form With Other Atoms In A Compound?
Web bromine is capable of forming one bond when it is in its elemental form. Web typically, the atoms of group 4a form 4 covalent bonds; Boron can form a fourth covalent bond and thus acquire a formal negative charge. Web 9) based on your answer to question 7, explain why h, f, cl and br are most likely to be end (terminal) atoms rather than central (bridging) atoms in a molecule.
Modeling Lonic And Covalent Bonds Part 1:
In the case of dibromine, the combined. However, it can also form multiple bonds with other elements. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence. Web bromine will normally form one covalent bond.
Typically, Boron Forms 3 Covalent Bonds.
How many bonds does br form. Web how many rings does jupiter have; The bond ratio formally expresses the ratio of the bond issued to the company's. •br br submit your choice.
Group 6A Form 2 Bonds;
For example, it can form two bonds with. Basically 3 lone pairs on each br. 3) how many bonds does each s atom form? Members of a group typically have similar properties and electron configurations in their outer shell.