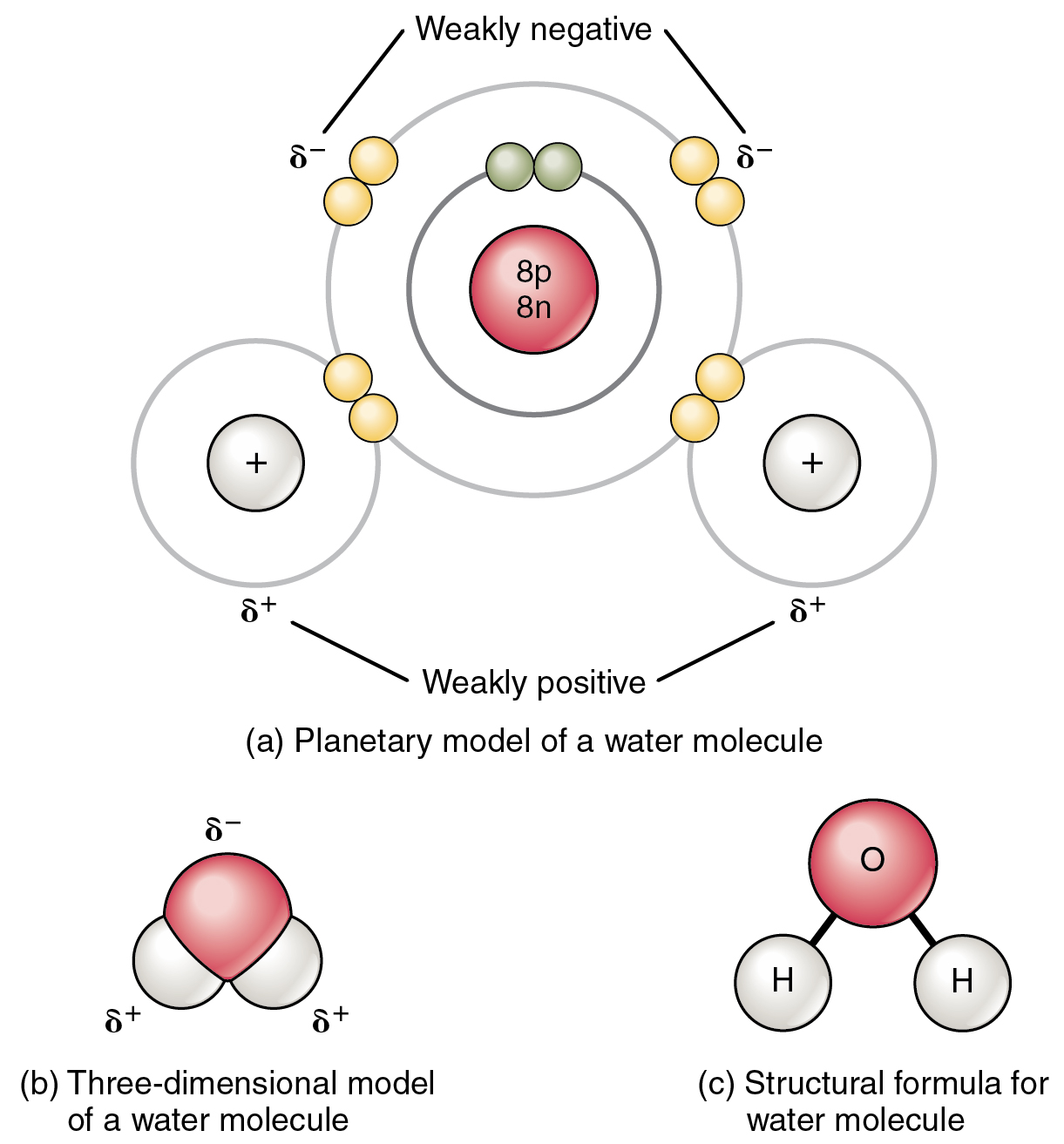
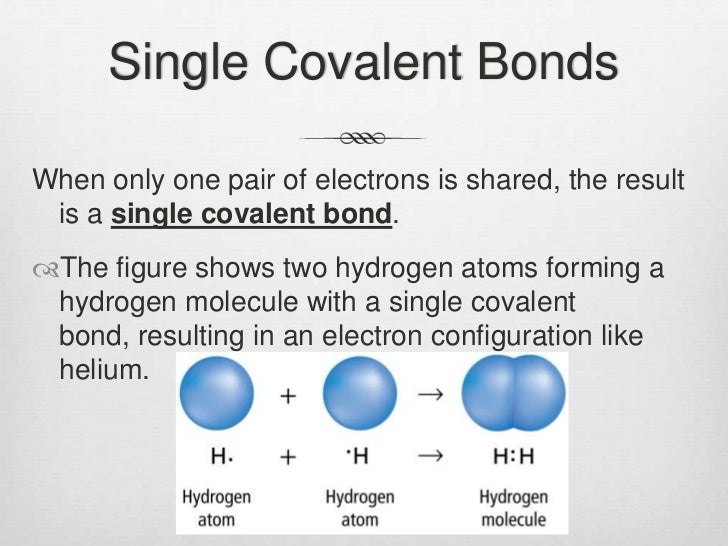
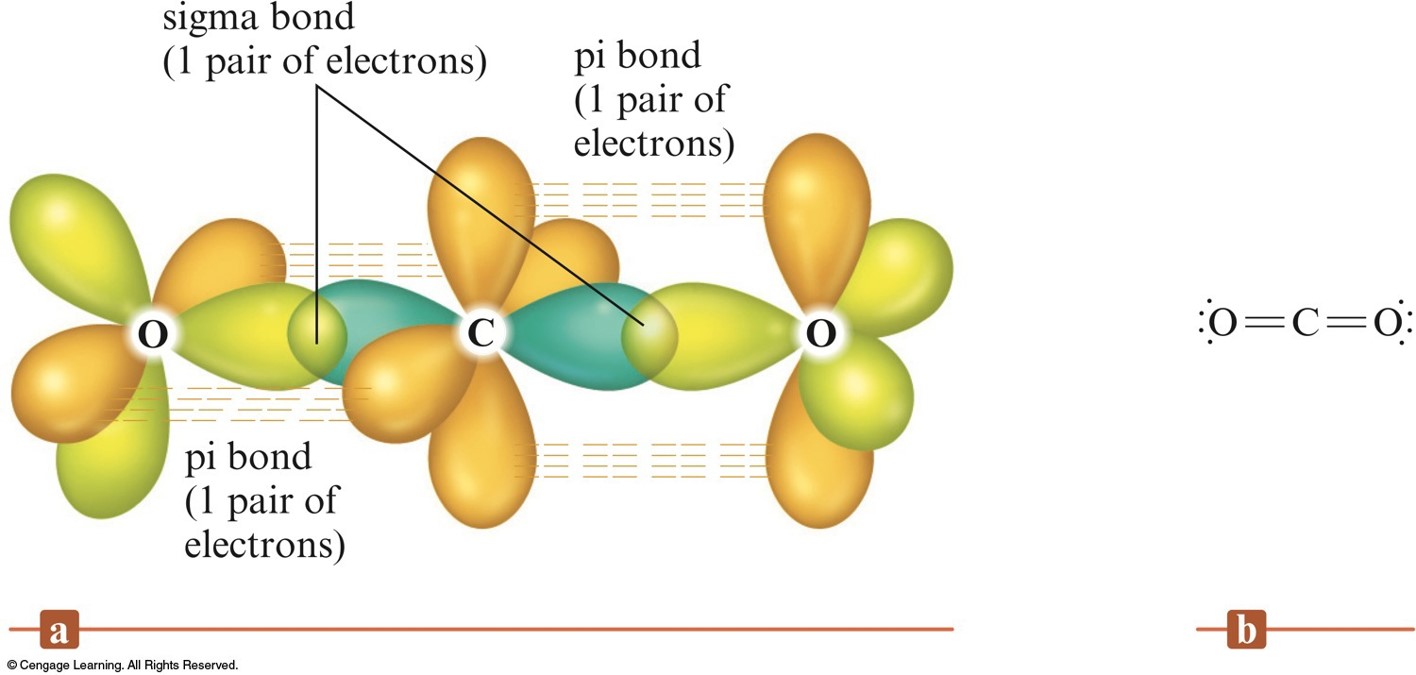
How Many Bonds Can N Form
How Many Bonds Can N Form - The number of electrons required to obtain. Group 5a form 3 bonds; Web both cl and n form the expected number of bonds. The electrons involved are in the outer shells of the atoms. H c n f 2. Web typically, the atoms of group 4a form 4 covalent bonds; Web for covalent bonds, it can form two single bonds or one double bond. Web but you can approximate the yield to maturity with the following shortcut formula: The central atom n (group 5a) has 3 bonds and one lone pair. Web generally, the greater the risk, the higher the interest paid by a bond.
The central atom n (group 5a) has 3 bonds and one lone pair. And group 7a form one bond. This is called the valence shell. Web both cl and n form the expected number of bonds. Carbon form generally covalent bonds; How many types of bonds can atom form? In general, it depends on how many electrons it has or are missing in the outermost electron shell. How many bonds does each of the following atoms normally form in molecules? H c n f 2. Web chemistry chemistry questions and answers 1.
Group 5a form 3 bonds; Web study with quizlet and memorize flashcards containing terms like how many bonds can n form?, primary amine, secondary amine and more. Group 6a form 2 bonds; N 2 is a stable (that is relatively unreactive) molecular compound. The electrons involved are in the outer shells of the atoms. Web the two n atoms are bonded together by a triple bond, consisting of a σ and two π bonds. Draw dot diagrams for s and for. Cl (group 7a) has one bond and 3 lone pairs. In general, it depends on how many electrons it has or are missing in the outermost electron shell. Group 6a form 2 bonds;
__TOP__ How Many Covalent Bonds Can Chlorine Form
Annual interest + annually accumulated discount/ average of par value and. Web how many bonds can an atom form? Web the two n atoms are bonded together by a triple bond, consisting of a σ and two π bonds. Group 6a form 2 bonds; Group 6a form 2 bonds;
Chemical Bonds Anatomy and Physiology I
Web generally, the greater the risk, the higher the interest paid by a bond. How many types of bonds can atom form? N 2 is a stable (that is relatively unreactive) molecular compound. The number of electrons required to obtain. Annual interest + annually accumulated discount/ average of par value and.
Chemical Bonds · Anatomy and Physiology
Web study with quizlet and memorize flashcards containing terms like how many bonds can n form?, primary amine, secondary amine and more. Hydrogen has one electron in its. In general, it depends on how many electrons it has or are missing in the outermost electron shell. N 2 is a stable (that is relatively unreactive) molecular compound. This is called.
How many covalent bonds can hydrogen form?
And group 7a form one bond. Draw dot diagrams for s and for. This is called the valence shell. Annual interest + annually accumulated discount/ average of par value and. How many bonds does each of the following atoms normally form in molecules?
metallic bond Properties, Examples, & Explanation Britannica
And group 7a form one bond. Web generally, the greater the risk, the higher the interest paid by a bond. This is called the valence shell. Web for covalent bonds, it can form two single bonds or one double bond. Web but you can approximate the yield to maturity with the following shortcut formula:
The 4 Types of Bonds Carbon Can Form Video & Lesson Transcript
Group 5a form 3 bonds; N 2 is a stable (that is relatively unreactive) molecular compound. Web study with quizlet and memorize flashcards containing terms like how many bonds can n form?, primary amine, secondary amine and more. This is called the valence shell. How many types of bonds can atom form?
Reading Covalent Bonds Biology I
This is called the valence shell. N 2 is a stable (that is relatively unreactive) molecular compound. Group 5a form 3 bonds; Web generally, the greater the risk, the higher the interest paid by a bond. And group 7a form one bond.
How many pi bonds can the atom form? Socratic
Cl (group 7a) has one bond and 3 lone pairs. Web typically, the atoms of group 4a form 4 covalent bonds; Group 6a form 2 bonds; And group 7a form one bond. Web both cl and n form the expected number of bonds.
polarity Definition & Examples Britannica
Web study with quizlet and memorize flashcards containing terms like how many bonds can n form?, primary amine, secondary amine and more. The electrons involved are in the outer shells of the atoms. Cl (group 7a) has one bond and 3 lone pairs. Recognising where there are lone pairs that. Hydrogen has one electron in its.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
The electrons involved are in the outer shells of the atoms. Carbon form generally covalent bonds; N 2 is a stable (that is relatively unreactive) molecular compound. And group 7a form one bond. Web as for the formation of a single covalent bond, one electron is shared by each atom, thus nitrogen can share its three electrons to form 3.
How Many Bonds Does Each Of The Following Atoms Normally Form In Molecules?
N 2 is a stable (that is relatively unreactive) molecular compound. Group 6a form 2 bonds; This is called the valence shell. Group 6a form 2 bonds;
Web As For The Formation Of A Single Covalent Bond, One Electron Is Shared By Each Atom, Thus Nitrogen Can Share Its Three Electrons To Form 3 Single Covalent Bonds.
Annual interest + annually accumulated discount/ average of par value and. Web typically, the atoms of group 4a form 4 covalent bonds; Web typically, the atoms of group 4a form 4 covalent bonds; And group 7a form one bond.
Web Both Cl And N Form The Expected Number Of Bonds.
And group 7a form one bond. Group 5a form 3 bonds; Hydrogen has one electron in its. How many types of bonds can atom form?
The Electrons Involved Are In The Outer Shells Of The Atoms.
Web generally, the greater the risk, the higher the interest paid by a bond. Cl (group 7a) has one bond and 3 lone pairs. The number of electrons required to obtain. Web for covalent bonds, it can form two single bonds or one double bond.