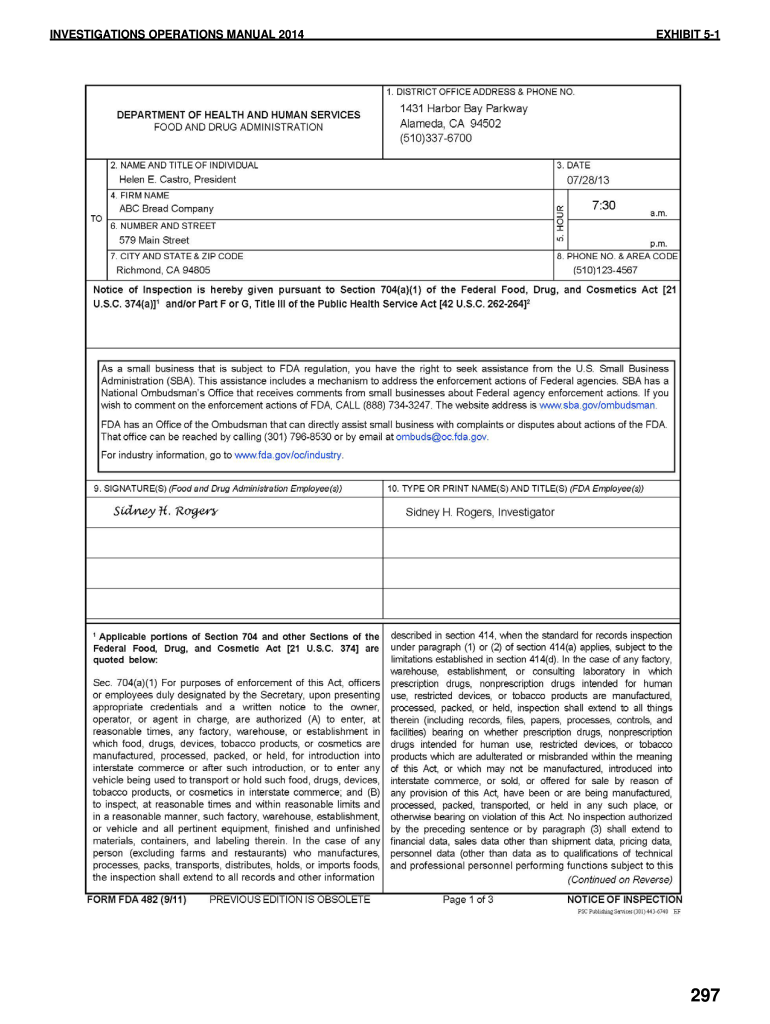
Fda 482 Form
Fda 482 Form - Ad edit, fill & esign pdf documents online. (a) manufacturers, distributors, multiple distributors, and final distributors shall, upon the. Food and drug administration office of regulatory affairs field operations: Web get your online template and fill it in using progressive features. As per food and drug cosmetic act. You can also download it, export it or print it out. Experience all the benefits of. Web fda 482 notice of inspection. Follow the simple instructions below: Fda investigators must formally identify themselves by presenting credentials;
Web the investigator will also request fsvp records in writing (form fda 482d). Web send form fda 482 via email, link, or fax. Ad edit, fill & esign pdf documents online. Edit your fda form 482 online. During an usfda inspection, ora investigators may observe conditions they deem to be objectionable. Follow the simple instructions below: An fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a. Web risk follow up inspections to a regulatory action complaints (public & industry) what is high priority for inspection? Depending on the browser you are using, you may need to download the form to enable field fillable functionality. As per food and drug cosmetic act.
Fda form 482 is called a notice of inspection form. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. During an usfda inspection, ora investigators may observe conditions they deem to be objectionable. Web risk follow up inspections to a regulatory action complaints (public & industry) what is high priority for inspection? (a) manufacturers, distributors, multiple distributors, and final distributors shall, upon the. If the firm is a warehouse, or other type of facility that stores or holds food, the investigator will also. Web • fda form 482c. Follow the simple instructions below: Web get your online template and fill it in using progressive features. These observations, are listed on an fda form 483 when, in.
RosaceaLtd applies for FDA Approval RosaceaLtd IV
Follow the simple instructions below: An fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a. Fda form 482 is called a notice of inspection form. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. As per food and.
A cGMP Primer
Web • fda form 482c. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Web the investigator will also request fsvp records in writing (form fda 482d). Web risk follow up inspections to a regulatory action complaints (public & industry) what is high priority for inspection? You can also download.
PPT Patricia Kerby, MPA Human Subjections Protection Compliance
Best pdf fillable form builder. (a) manufacturers, distributors, multiple distributors, and final distributors shall, upon the. During an usfda inspection, ora investigators may observe conditions they deem to be objectionable. Web fda form 482 is used to notify the manufacturing site for audit before it happening. Depending on the browser you are using, you may need to download the form.
A cGMP Primer
Web also known as a notice of inspection, the food and drug administration (fda) form 482 is an official document presented to the investigator upon arrival at the. An fda 482 may conduct an inspection of your operation for a variety of reasons, such as a routinely scheduled investigation, a. Edit your fda form 482 online. Draw your signature, type.
corrective action plan example in 2021 How to plan, Graduation card
Regional/district offices who conducts inspections for fda? Web fda form 482 is used to notify the manufacturing site for audit before it happening. Official fda inspection form completed by fda investigators and presented to the most responsible person (such as the principal. Web get your online template and fill it in using progressive features. Web send form fda 482 via.
Form 482 Fill Online, Printable, Fillable, Blank pdfFiller
Web also known as a notice of inspection, the food and drug administration (fda) form 482 is an official document presented to the investigator upon arrival at the. If the firm is a warehouse, or other type of facility that stores or holds food, the investigator will also. Web otice of inspection (fda form 482): Web fda form 482 is.
what is FDA 482 and FDA 484 and other form used in FDA inspection Key
Follow the simple instructions below: Web understanding fda inspection & form 482 | form 483 4.60 (5 ratings) understanding fda inspection & form 482 | form 483 categories free manufacturing course, free. Ad edit, fill & esign pdf documents online. Type text, add images, blackout confidential details, add comments, highlights and more. Web the investigator will also request fsvp records.
Form FDA 3613b Supplementary Information Certificate of a
Web the investigator will also request fsvp records in writing (form fda 482d). Food and drug administration office of regulatory affairs field operations: These observations, are listed on an fda form 483 when, in. Fda form 482 is called a notice of inspection form. Enjoy smart fillable fields and interactivity.
Form FDA 3613a Supplementary Information Certificate of Exportability
You can also download it, export it or print it out. Best pdf fillable form builder. Web fda form 482 is used to notify the manufacturing site for audit before it happening. Type text, add images, blackout confidential details, add comments, highlights and more. Follow the simple instructions below:
If The Firm Is A Warehouse, Or Other Type Of Facility That Stores Or Holds Food, The Investigator Will Also.
Sign it in a few clicks. Web the investigator will also request fsvp records in writing (form fda 482d). Follow the simple instructions below: Fda form 482 is called a notice of inspection form.
Edit Your Fda Form 482 Online.
Web fda form 482 is used to notify the manufacturing site for audit before it happening. You can also download it, export it or print it out. (a) manufacturers, distributors, multiple distributors, and final distributors shall, upon the. Web send form fda 482 via email, link, or fax.
Make Class Iii Or Class Ii Devices.
Web risk follow up inspections to a regulatory action complaints (public & industry) what is high priority for inspection? Edit your fda 482 blank online. Official fda inspection form completed by fda investigators and presented to the most responsible person (such as the principal. Web • fda form 482c.
Enjoy Smart Fillable Fields And Interactivity.
Best pdf fillable form builder. During an usfda inspection, ora investigators may observe conditions they deem to be objectionable. Draw your signature, type it,. Web understanding fda inspection & form 482 | form 483 4.60 (5 ratings) understanding fda inspection & form 482 | form 483 categories free manufacturing course, free.