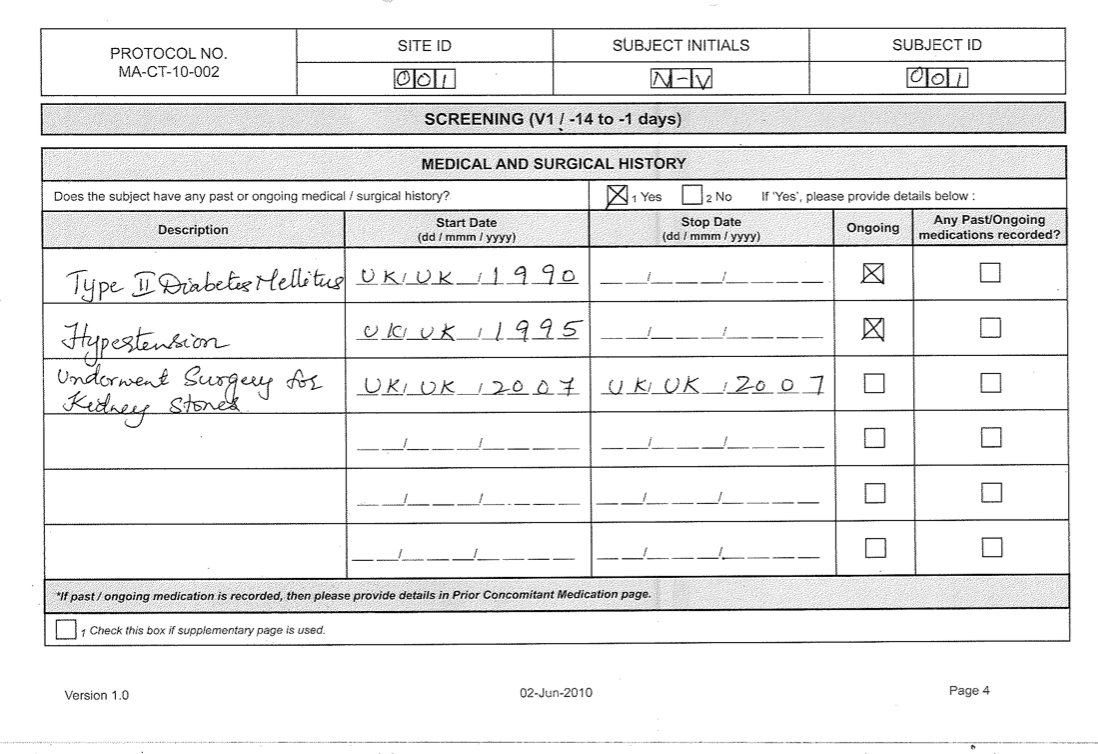
Crf Full Form In Clinical Research
Crf Full Form In Clinical Research - 2 meanings of crf abbreviation related to research: Crfs play a crucial role in helping to assess the safety and efficacy of clinical products. Case report form (crf) is a specialized document in clinical research. A form which is used for capturing data in pharmaceutical and medical device clinical trials. [ 1] site personnel capture the subject's data on the crf, which is collected during their participation in a clinical trial. It should be study protocol driven, robust in content and have material to collect the study specific data. Its development represents a significant part of the clinical trial and can affect study success. Common terminology criteria for adverse events. Web a case report form (crf) is designed to collect the patient data in a clinical trial; For a study to be successful, the data collected must be correct and complete.
Electronic crfs are powered by automation technology. [ 1] site personnel capture the subject's data on the crf, which is collected during their participation in a clinical trial. 2 meanings of crf abbreviation related to research: This data can include everything from participants’ personal information to study visit results to treatment outcomes and side effects experienced. A form which is used for capturing data in pharmaceutical and medical device clinical trials. Web crf is a commonly used acronym for case report form; Its development represents a significant part of the clinical trial and can affect study success. Web what is crf meaning in research? Common terminology criteria for adverse events. Web case report form (crf) is a key document for data collection in clinical trials.
A form which is used for capturing data in pharmaceutical and medical device clinical trials. Web crf is a commonly used acronym for case report form; Common terminology criteria for adverse events. It should be study protocol driven, robust in content and have material to collect the study specific data. Case report form (crf) is a specialized document in clinical research. Web the terms ‘crf’ and ‘ecrf’ tend to be used interchangeably, and they’re also referred to simply as ‘forms’. Web case report form (crf) refers to a form that collects patient data that investigators use during a visit. Electronic crfs are powered by automation technology. [ 1] site personnel capture the subject's data on the crf, which is collected during their participation in a clinical trial. Though paper crfs are still used largely, use of electronic crfs (ecrfs) are gaining popularity due to the advantages they offer such as improved data.
Form E Report E2 80 93 Riat Support Center Crf Templates Intended For
It should be study protocol driven, robust in content and have material to collect the study specific data. Its development represents a significant part of the clinical trial and can affect study success. A form which is used for capturing data in pharmaceutical and medical device clinical trials. Crfs play a crucial role in helping to assess the safety and.
Query Management in Field Research (Beginner's Guide)
A form which is used for capturing data in pharmaceutical and medical device clinical trials. Crfs play a crucial role in helping to assess the safety and efficacy of clinical products. It should be study protocol driven, robust in content and have material to collect the study specific data. Web the terms ‘crf’ and ‘ecrf’ tend to be used interchangeably,.
CRF Full Form In Hindi CRF Se Kya Samjhte Hai PagalNews
Web what is crf meaning in research? Electronic crfs are powered by automation technology. A form which is used for capturing data in pharmaceutical and medical device clinical trials. Web case report form (crf) is a key document for data collection in clinical trials. This data can include everything from participants’ personal information to study visit results to treatment outcomes.
PPT SAFETY MONITORING IN CLINICAL TRIALS PowerPoint Presentation
Crfs play a crucial role in helping to assess the safety and efficacy of clinical products. Case report forms (crfs) have historically always been on something like carbon copy paper, so one sheet can be added to the trial master file (tmf, another acronym to look out for!). Web case report form (crf) refers to a form that collects patient.
Case Report Form (CRF) for clinical examination 1. CRF to be filled by
[ 1] site personnel capture the subject's data on the crf, which is collected during their participation in a clinical trial. Web crf is a commonly used acronym for case report form; Web what is crf meaning in research? Its development represents a significant part of the clinical trial and can affect study success. Common terminology criteria for adverse events.
Full form of CRF Relejuvant Clinical Services
Case report form (crf) is a specialized document in clinical research. Web what is crf meaning in research? Its development represents a significant part of the clinical trial and can affect study success. Web crf is a commonly used acronym for case report form; Web case report form (crf) is a key document for data collection in clinical trials.
Longterm followup form. Simple one page CRF for collection of primary
Web case report form (crf) refers to a form that collects patient data that investigators use during a visit. Web crf is a commonly used acronym for case report form; Crfs play a crucial role in helping to assess the safety and efficacy of clinical products. For a study to be successful, the data collected must be correct and complete..
Usoda Monumentalno analogija case report form poglej izgovorjava Opis
Case report forms (crfs) have historically always been on something like carbon copy paper, so one sheet can be added to the trial master file (tmf, another acronym to look out for!). Its development represents a significant part of the clinical trial and can affect study success. Common terminology criteria for adverse events. Web what is crf meaning in research?.
Usoda Monumentalno analogija case report form poglej izgovorjava Opis
2 meanings of crf abbreviation related to research: This data can include everything from participants’ personal information to study visit results to treatment outcomes and side effects experienced. Electronic crfs are powered by automation technology. Though paper crfs are still used largely, use of electronic crfs (ecrfs) are gaining popularity due to the advantages they offer such as improved data..
Form Crf004 Additional Ownership / Relationship Form printable pdf
Web crf is a commonly used acronym for case report form; Though paper crfs are still used largely, use of electronic crfs (ecrfs) are gaining popularity due to the advantages they offer such as improved data. Crfs play a crucial role in helping to assess the safety and efficacy of clinical products. It should be study protocol driven, robust in.
Web Case Report Form (Crf) Is A Key Document For Data Collection In Clinical Trials.
This data can include everything from participants’ personal information to study visit results to treatment outcomes and side effects experienced. Crfs play a crucial role in helping to assess the safety and efficacy of clinical products. Electronic crfs are powered by automation technology. Case report form (crf) is a specialized document in clinical research.
2 Meanings Of Crf Abbreviation Related To Research:
Common terminology criteria for adverse events. For a study to be successful, the data collected must be correct and complete. A form which is used for capturing data in pharmaceutical and medical device clinical trials. Though paper crfs are still used largely, use of electronic crfs (ecrfs) are gaining popularity due to the advantages they offer such as improved data.
Its Development Represents A Significant Part Of The Clinical Trial And Can Affect Study Success.
Web the terms ‘crf’ and ‘ecrf’ tend to be used interchangeably, and they’re also referred to simply as ‘forms’. Web crf is a commonly used acronym for case report form; [ 1] site personnel capture the subject's data on the crf, which is collected during their participation in a clinical trial. Case report forms (crfs) have historically always been on something like carbon copy paper, so one sheet can be added to the trial master file (tmf, another acronym to look out for!).
It Should Be Study Protocol Driven, Robust In Content And Have Material To Collect The Study Specific Data.
Web case report form (crf) refers to a form that collects patient data that investigators use during a visit. Web what is crf meaning in research? Web a case report form (crf) is designed to collect the patient data in a clinical trial;