Conceptual Physics Practice Page Chapter 14 Gases Gas Pressure Answers
Conceptual Physics Practice Page Chapter 14 Gases Gas Pressure Answers - Web 1 / 6 the pressure exerted against bodies immersed in the atmosphere. Web if the number of gas atoms in a container is doubled, the pressure of the gas doubles (assuming constant temperature and volume). For more practice, at the end of each chapter. Explain this pressure increase in terms of the molecular motion of the gas. Wrong choice in the context of the problem. Explain this pressure increase in terms of the molecular motion of the gas. Click the card to flip 👆 flashcards. Explain this pressure increase in terms of molecular motion of the gas It results from the weight of air pressing down from above. Web conceptual physics chapter 14:
It results from the weight of air pressing down from above. In general, atmospheric pressure affects fluid pressure unless the fluid is enclosed in a rigid container. Explain this pressure increase in terms of the molecular motion of the gas. Web atmospheric pressure does not affect the gas pressure in a rigid tank, but it does affect the pressure inside a balloon. If very little gravity (eg on moon), then molecules. A principle difference between a liquid and a gas is that when aliquid is under pressure, its volume. Kinetic energy of molecules vs gravity spreads molecules apart holds molecules near earth consider extremes: Web if the number of gas atoms in a container is doubled, the pressure of the gas doubles (assuming constant temperature and volume). Web conceptual physics chapter 14: Practice page chapter 14 gases and plasmas gas pressure date 1.
Web verified answer physics in an oscillating rlc circuit with $l = 10 \mathrm { mh } , c = 1.5 \mu \mathrm { f } , \text { and } r = 2.0 \omega$, how much time elapses before the amplitude of the oscillations drops to half. A principle difference between a liquid and a gas is that when a liquid is under pressure, its volume [increases]. A principle difference between a liquid and a gas is that when aliquid is under pressure, its volume. If very little gravity (eg on moon), then molecules. The density of a gas is the ratio of its weight to its volume that is density of a gas is inversely proportional to its volume. The main difference between a liquid and a gas is that when a liquid is under pressure… Gases study play atmospheric pressure the pressure caused by the weight of air barometer a device that measures air pressure boyle's law p₁v₁=p₂v₂ principle of. At sea level, atmospheric pressure is about 101 kpa. 70 date name conceptual physics practice page chapter 14 gases gas pressure 1. 4) density = molar mass (m) multiplied by p (pressure) over rt, where r is the gas constant and t is temperature.
Chapter 14 Gases
For more practice, at the end of each chapter. Web according to boyle’s law, the pressure will increase to three times its original pressure. Web if the number of gas atoms in a container is doubled, the pressure of the gas doubles (assuming constant temperature and volume). A principle difference between a liquid and a gas is that when aliquid.
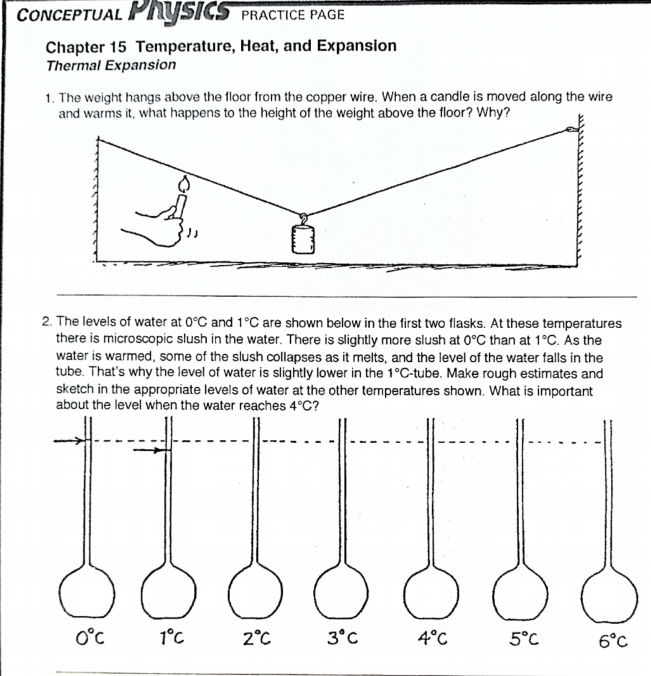
Solved CONCEPTUAL PHYSICS PRACTICE PAGE Chapter 15
Gases study play atmospheric pressure the pressure caused by the weight of air barometer a device that measures air pressure boyle's law p₁v₁=p₂v₂ principle of. Web test match created by dcrump78 plus terms in this set (5) atmospheric pressure force exerted by the weight of the air barometer an instrument that measures atmospheric pressure boyle's law a principle that describes.
Solved CONCEPTUAL Physics PRACTICE PAGE Chapter 17 Change of
In general, atmospheric pressure affects fluid pressure unless the fluid is enclosed in a rigid container. It results from the weight of air pressing down from above. Web 1 / 6 the pressure exerted against bodies immersed in the atmosphere. 70 date name conceptual physics practice page chapter 14 gases gas pressure 1. Gases study play atmospheric pressure the pressure.
Conceptual Physics Chapter 26 Exercises Answers ExerciseWalls
Web atmospheric pressure does not affect the gas pressure in a rigid tank, but it does affect the pressure inside a balloon. The pressure increases, in accord with boyle’s law. A moving molecule encountering a surface imparts force to the. Web physics chapter 14 gases atmosphere atmospheric pressure the highest the atmosphere can push wat… the maximum height to which.
Chapter 14 Gases
For more practice, at the end of each chapter. A principle difference between a liquid and a gas is that when aliquid is under pressure, its volume. Web conceptual physics chapter 14: Web atmospheric pressure does not affect the gas pressure in a rigid tank, but it does affect the pressure inside a balloon. Web if the number of gas.
Conceptual Physics Worksheet Answers wiildcreative
If very little gravity (eg on moon), then molecules. Web 14 gases conceptual physics instructor’s manual, 12th edition 14.1 the atmosphere 14.2 atmospheric pressure barometer 14.3 boyle’s law 14.4 buoyancy of air 14.5 bernoulli’s principle applications of bernoulli’s principle 14… Explain this pressure increase in terms of the molecular motion of the gas. Web verified answer physics in an oscillating.
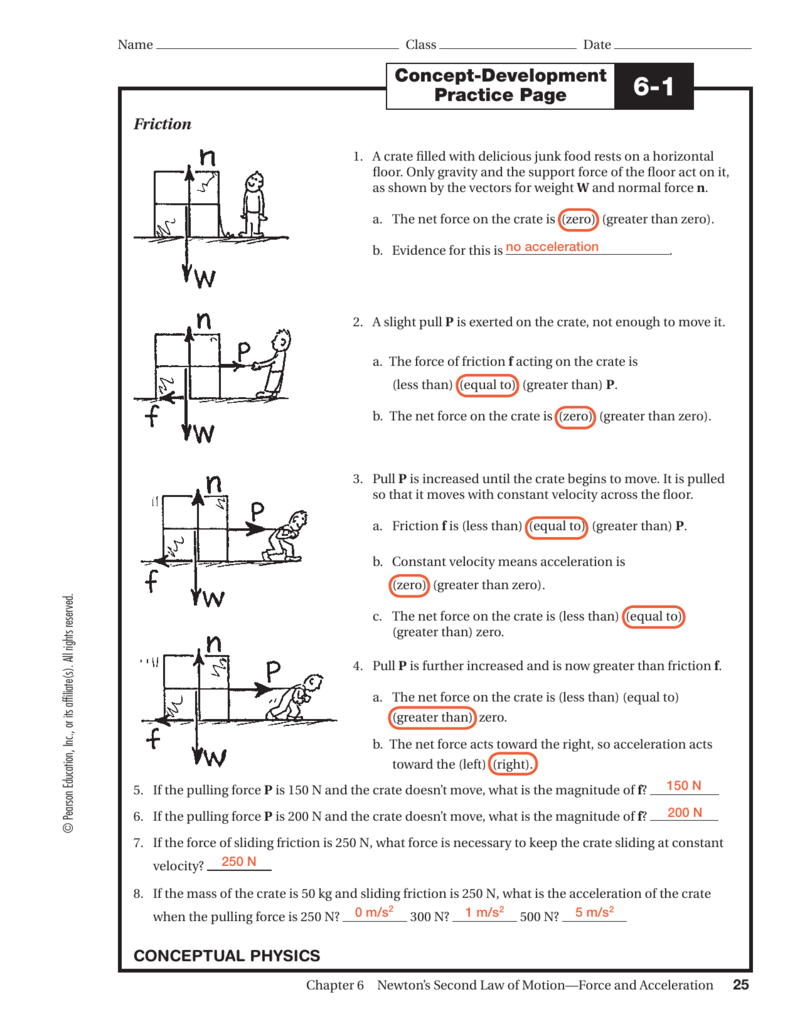
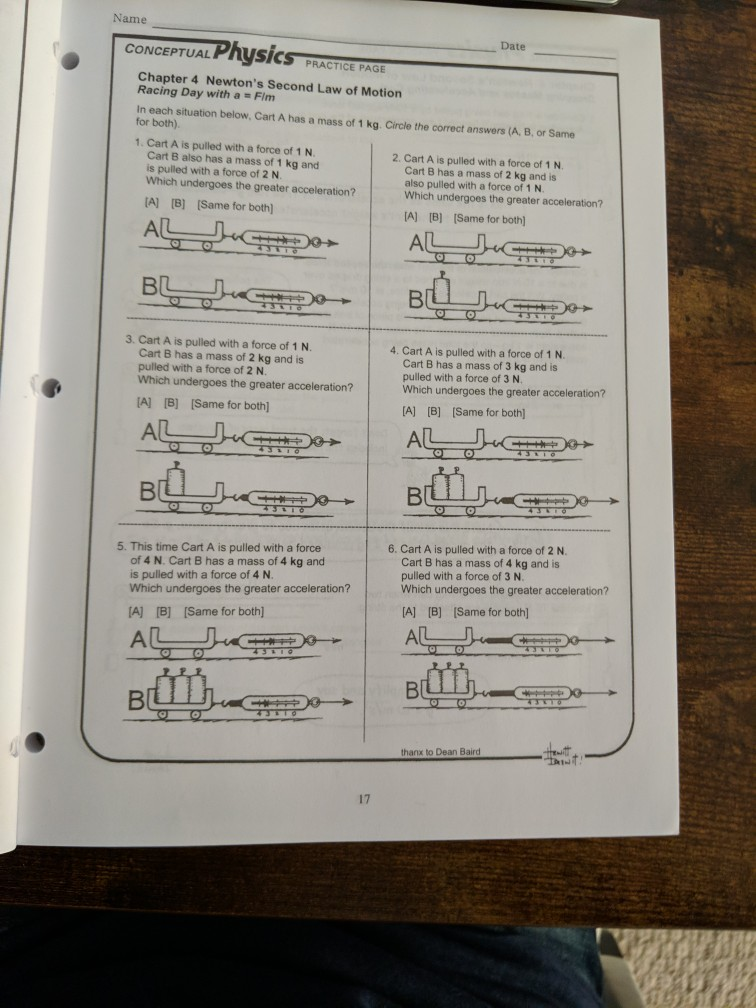
Solved Date Name CONCEPTUAL Physics PRACTICE PAGE Chapter 4
Web physics chapter 14 gases atmosphere atmospheric pressure the highest the atmosphere can push wat… the maximum height to which water can b… an ocean of air and exerts pressure. Web atmospheric pressure does not affect the gas pressure in a rigid tank, but it does affect the pressure inside a balloon. In general, atmospheric pressure affects fluid pressure unless.
Solved Name Physics Date CONCEPTUAL PRACTICE PAGE Chapter 4
For more practice, at the end of each chapter. Explain this pressure increase in terms of the molecular motion of the gas. Explain this pressure increase in terms of the molecular motion of the gas. Web if the number of gas atoms in a container is doubled, the pressure of the gas doubles (assuming constant temperature and volume). Explain this.
CONCEPTUAL PHYSICS Chapter 14 Gases Force, Area,
Web if the number of gas atoms in a container is doubled, the pressure of the gas doubles (assuming constant temperature and volume). The density of a gas is the ratio of its weight to its volume that is density of a gas is inversely proportional to its volume. A principle difference between a liquid and a gas is that.
CONCEPTUAL Physics PRACTICE PAGE Chapter 8 Rotational
A principle difference between a liquid and a gas is that when aliquid is under pressure, its volume. Web atmospheric pressure does not affect the gas pressure in a rigid tank, but it does affect the pressure inside a balloon. Web physics chapter 14 gases atmosphere atmospheric pressure the highest the atmosphere can push wat… the maximum height to which.
Web 3) P Is Directly Proportional To N.
The main difference between a liquid and a gas is that when a liquid is under pressure… Web the atmosphere what determines the thickness of our atmosphere? A principle difference between a liquid and a gas is that when a liquid is under pressure, its volume [increases]. It results from the weight of air pressing down from above.
Explain This Pressure Increase In Terms Of The Molecular Motion Of The Gas.
Web test match created by dcrump78 plus terms in this set (5) atmospheric pressure force exerted by the weight of the air barometer an instrument that measures atmospheric pressure boyle's law a principle that describes the relationship between the pressure and volume of a gas. Gases study play atmospheric pressure the pressure caused by the weight of air barometer a device that measures air pressure boyle's law p₁v₁=p₂v₂ principle of. Web physics chapter 14 gases atmosphere atmospheric pressure the highest the atmosphere can push wat… the maximum height to which water can b… an ocean of air and exerts pressure. A moving molecule encountering a surface imparts force to the.
70 Date Name Conceptual Physics Practice Page Chapter 14 Gases Gas Pressure 1.
Wrong choice in the context of the problem. Web if the number of gas atoms in a container is doubled, the pressure of the gas doubles (assuming constant temperature and volume). Web if the number of gas atoms in a container is doubled, the pressure of the gas doubles (assuming constant temperature and volume). Web if the number of gas atoms in a container is doubled, the pressure of the gas doubles (assuming constant temperature and volume).
Web Verified Answer Physics In An Oscillating Rlc Circuit With $L = 10 \Mathrm { Mh } , C = 1.5 \Mu \Mathrm { F } , \Text { And } R = 2.0 \Omega$, How Much Time Elapses Before The Amplitude Of The Oscillations Drops To Half.
The pressure increases, in accord with boyle’s law. In general, atmospheric pressure affects fluid pressure unless the fluid is enclosed in a rigid container. If very little gravity (eg on moon), then molecules. Web 1 / 6 the pressure exerted against bodies immersed in the atmosphere.