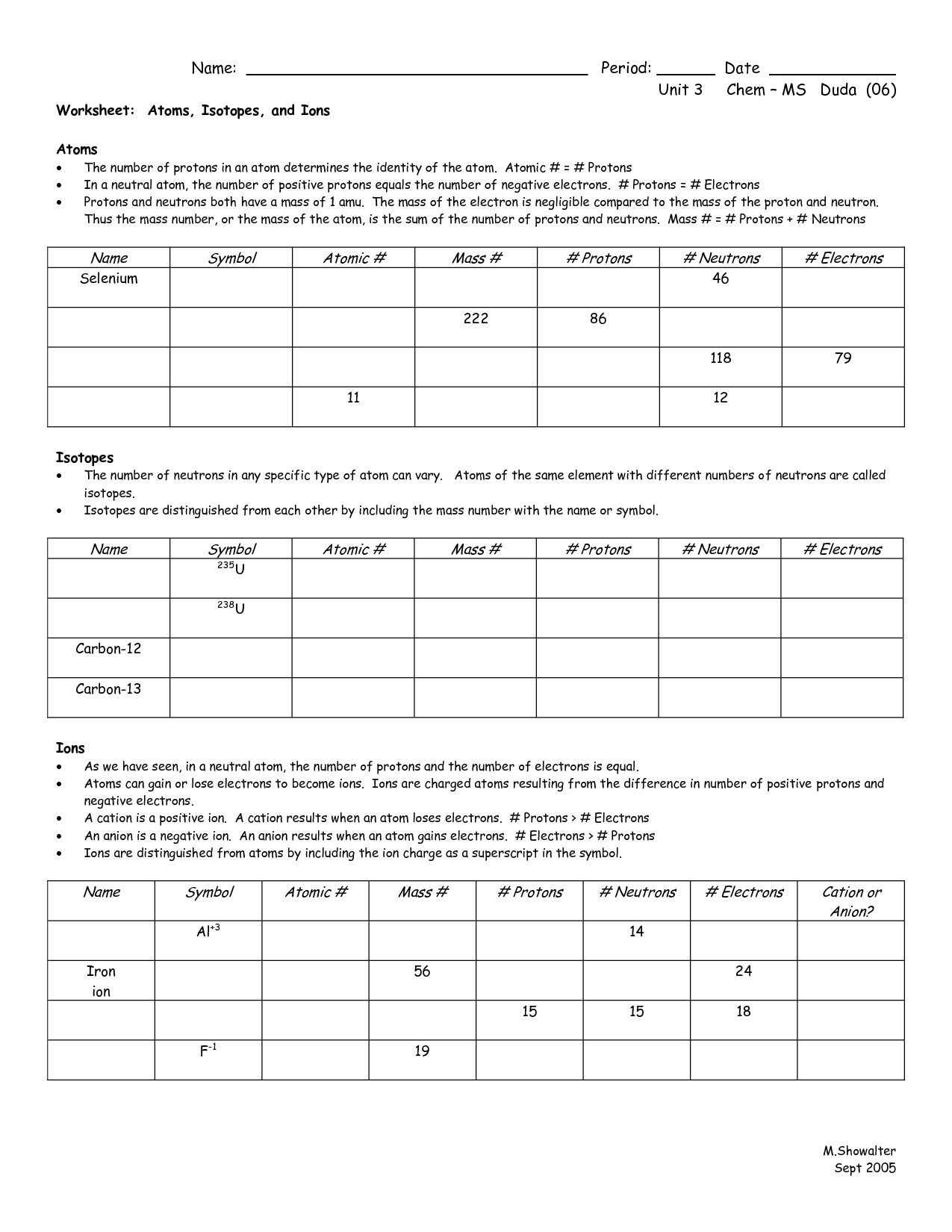
Chemistry Chapter 5 Electrons In Atoms Answer Key
Chemistry Chapter 5 Electrons In Atoms Answer Key - Learn vocabulary, terms and more with flashcards, games and other study tools. A hot object would emit electromagnetic energy in a continuous fashion. Web this online broadcast electrons in atoms chapter 5 answer key can be one of the options to accompany you considering. The periodic law states that. Web 32 terms · n= → number of sub levels, n^2 → number of orbitals, 2n^2 → number of electrons in each en…, energy level is →. Web test match created by chloecourant terms in this set (27) why was rutherford's model of the atom known as the planetary. The most valence electrons for any element is 8. Web amplitude ____ is the _______, which is equivalent to one wave per second. Electrons in atoms 5.1 properties of light check your understanding 1. You may have made it through the first four chapters, but today.
Both are orbitals that can contain two electrons. Web start studying chapter 5: The height of a wave from the origin to a crest,or from the origin to a trough. Electrons in atoms 5.1 properties of light check your understanding 1. No two electrons with the same spin can be found in the. Web test match created by chloecourant terms in this set (27) why was rutherford's model of the atom known as the planetary. A hot object would emit electromagnetic energy in a continuous fashion. Web 1 / 45 the si unit of frequency click the card to flip 👆 flashcards learn test match created by tanya_myers52 terms in this set (45). In the model mode, each electron group occupies the same amount of space, so the bond angle. Web amplitude ____ is the _______, which is equivalent to one wave per second.
Web chapter 5 electrons in atoms answers 5.3. Web this online broadcast electrons in atoms chapter 5 answer key can be one of the options to accompany you considering. The periodic law states that. No two electrons with the same spin can be found in the. A set of frequencies of electromagnetic waves given off by atoms of an element; Web 1 / 45 the si unit of frequency click the card to flip 👆 flashcards learn test match created by tanya_myers52 terms in this set (45). Web 32 terms · n= → number of sub levels, n^2 → number of orbitals, 2n^2 → number of electrons in each en…, energy level is →. Web the pauli exclusion principle, which states that no two electrons in an atom can have the same set of four. Web test match created by chloecourant terms in this set (27) why was rutherford's model of the atom known as the planetary. What are the general properties of light?
Chapter 5 electrons in atoms
Matter and change chapter 5: Both are orbitals that can contain two electrons. The most valence electrons for any element is 8. Web 1 / 45 the si unit of frequency click the card to flip 👆 flashcards learn test match created by tanya_myers52 terms in this set (45). Web each electron occupies the lowest energy orbital available, it is.
Chapter 5 Electrons In Atoms Answer Key THE PREMIER FEMALE DJ OF LOS
Web start studying chapter 5: No two electrons with the same spin can be found in the. Web the structures are very similar. The energy of the hot body. Atoms are made of extremely tiny particles called protons, neutrons, and electrons.
Chapter 5 Electrons In Atoms Answer Key THE PREMIER FEMALE DJ OF LOS
Atoms are made of extremely tiny particles called protons, neutrons, and electrons. Web this online broadcast electrons in atoms chapter 5 answer key can be one of the options to accompany you considering. What are the general properties of light? Web 32 terms · n= → number of sub levels, n^2 → number of orbitals, 2n^2 → number of electrons.
5 1 Light And Quantized Energy Worksheet Answers
Web start studying chapter 5: A set of frequencies of electromagnetic waves given off by atoms of an element; A hot object would emit electromagnetic energy in a continuous fashion. Web each electron occupies the lowest energy orbital available, it is fundamentally impossible to know precisely both the. Matter and change chapter 5:
Chapter 5 Electrons In Atoms Answer Key Chemistry
Web each electron occupies the lowest energy orbital available, it is fundamentally impossible to know precisely both the. A set of frequencies of electromagnetic waves given off by atoms of an element; The periodic law states that. Web chapter 5 electrons in atoms answers 5.3. Web 1 / 45 the si unit of frequency click the card to flip 👆.
Chapter 5 Assessment, solution manual,Electrons in Atoms, glencoe
The energy of the hot body. A hot object would emit electromagnetic energy in a continuous fashion. Web this online broadcast electrons in atoms chapter 5 answer key can be one of the options to accompany you considering. Both are orbitals that can contain two electrons. Learn vocabulary, terms and more with flashcards, games and other study tools.
10+ Chapter 5 Electrons In Atoms Answer Key WilliamMiran
Hertz, frequency a (n) _____ is the minimum. Web chemistry chapter 5 electrons in atoms term 1 / 51 difference between ground state and the excited state of an electron? The most valence electrons for any element is 8. In the model mode, each electron group occupies the same amount of space, so the bond angle. The height of a.
chapter 5 electrons in atoms chemistry
Electrons in atoms in this chapter: The energy of the hot body. Web test match created by chloecourant terms in this set (27) why was rutherford's model of the atom known as the planetary. Web chemistry chapter 5 quiz: Atoms are made of extremely tiny particles called protons, neutrons, and electrons.
Electrons In Atoms Worksheet Answers Chapter 5 Breadandhearth
Web chemistry chapter 5 quiz: Web test match created by chloecourant terms in this set (27) why was rutherford's model of the atom known as the planetary. Learn vocabulary, terms and more with flashcards, games and other study tools. Web chapter 5 electrons in atoms answers 5.3. Hertz, frequency a (n) _____ is the minimum.
16 Molecules And Atoms Worksheet Answer Key /
Web chemistry chapter 5 electrons in atoms term 1 / 51 difference between ground state and the excited state of an electron? Learn vocabulary, terms and more with flashcards, games and other study tools. The height of a wave from the origin to a crest,or from the origin to a trough. What are the general properties of light? The energy.
Web 1 / 45 The Si Unit Of Frequency Click The Card To Flip 👆 Flashcards Learn Test Match Created By Tanya_Myers52 Terms In This Set (45).
No two electrons with the same spin can be found in the. You may have made it through the first four chapters, but today. Web the pauli exclusion principle, which states that no two electrons in an atom can have the same set of four. A set of frequencies of electromagnetic waves given off by atoms of an element;
Web The Structures Are Very Similar.
Web this online broadcast electrons in atoms chapter 5 answer key can be one of the options to accompany you considering. Web start studying chapter 5: The periodic law states that. What are the general properties of light?
Atoms Are Made Of Extremely Tiny Particles Called Protons, Neutrons, And Electrons.
Web chemistry chapter 5 quiz: Web 32 terms · n= → number of sub levels, n^2 → number of orbitals, 2n^2 → number of electrons in each en…, energy level is →. In the model mode, each electron group occupies the same amount of space, so the bond angle. Both are orbitals that can contain two electrons.
Hertz, Frequency A (N) _____ Is The Minimum.
A hot object would emit electromagnetic energy in a continuous fashion. Web chapter 5 electrons in atoms answers 5.3. Web chemistry chapter 5 electrons in atoms term 1 / 51 difference between ground state and the excited state of an electron? Web test match created by chloecourant terms in this set (27) why was rutherford's model of the atom known as the planetary.