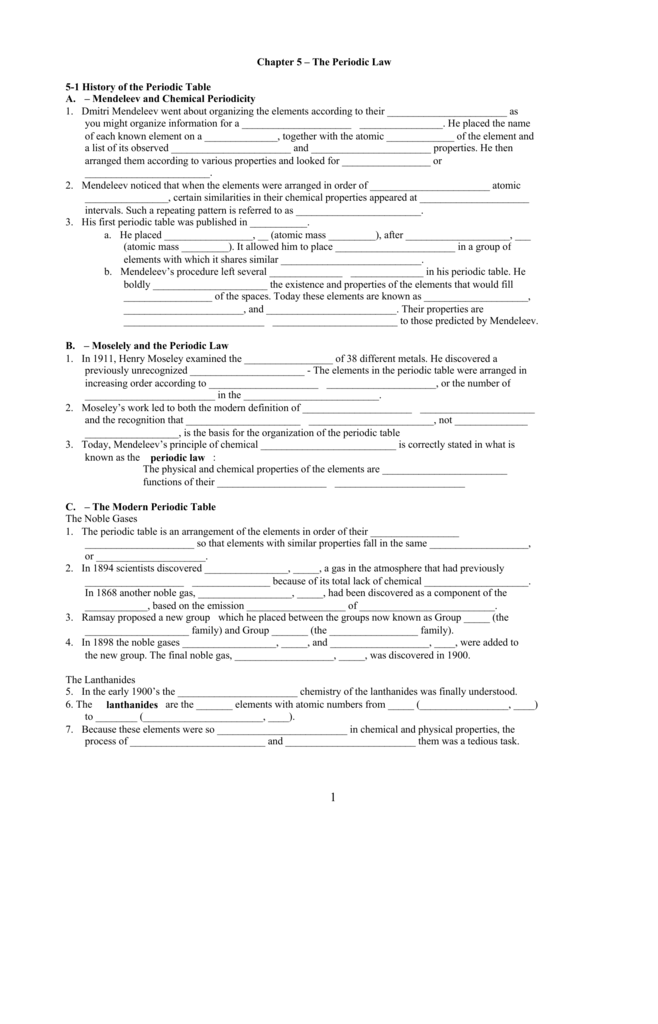
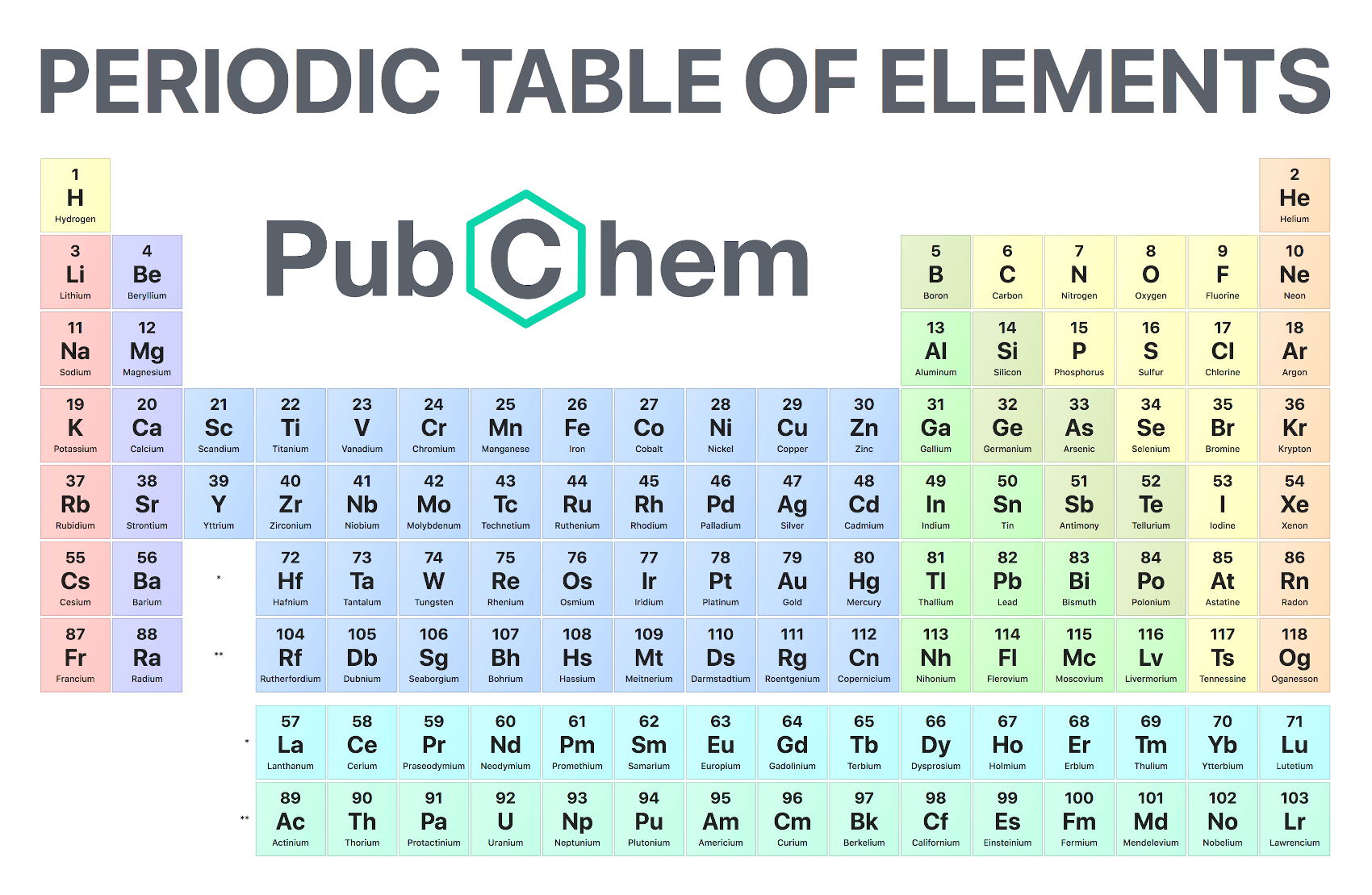
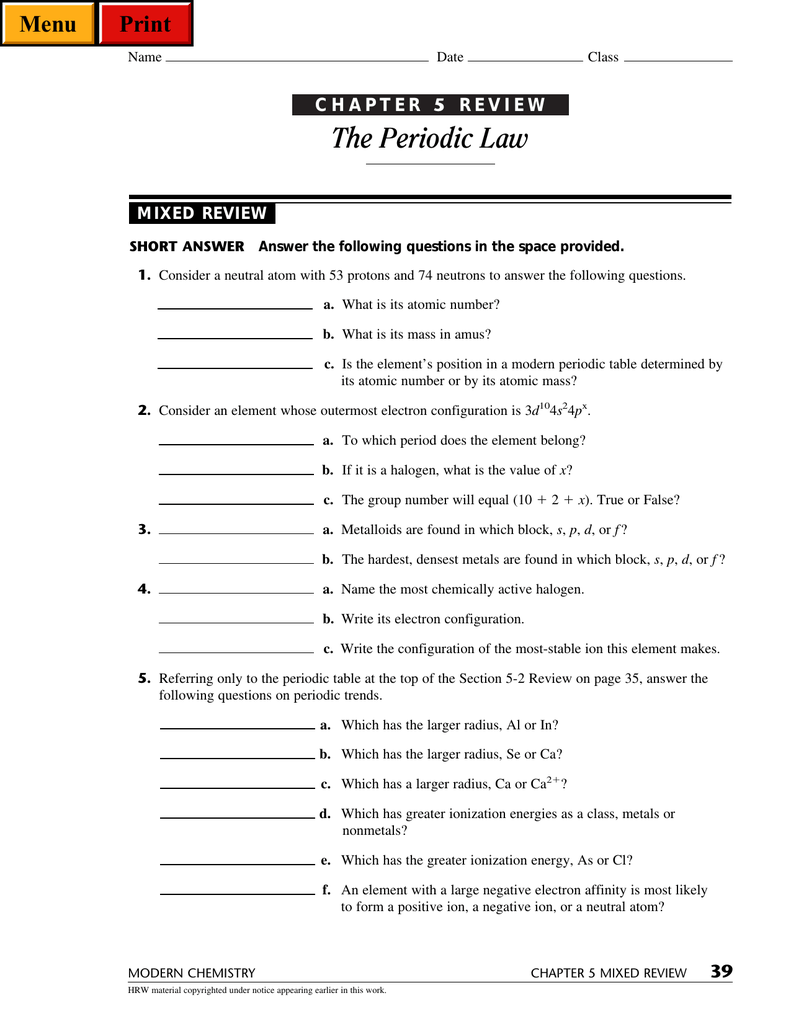
Chapter 5 The Periodic Law Answer Key
Chapter 5 The Periodic Law Answer Key - The radius of a positive ion is smaller than the radius of its neutral atom. (2 things) periodic law the physical and chemical properties of the elements are periodic functions of their atomic numbers periodic. Based on the reasons and the arguments presented in the case, i am not surprised that wedow v. Periodic table review answer key. Web periodic law big idea the properties of the chemical elements are a periodic function of their atomic numbers. Web holt mcdougal modern chemistry 1 the periodic law chapter 5 review the periodic law section 2 short answer use this periodic table to answer the following questions in the space. Are you surprised that this is a 2006 case? The physical and chemical properties of an element are functions of its atomic number. 2.) what can be removed from an atom is ionization energy is supplied. Web the periodic law section 1 answer the following questions in the space provided.
C in the modern periodic table, elements are ordered (a) according to decreasing atomic mass. Are you surprised that this is a 2006 case? (2 things) periodic law the physical and chemical properties of the elements are periodic functions of their atomic numbers periodic. Compare the radius of a negative ion to the radius of its neutral. The radius of a positive ion is smaller than the radius of its neutral atom. Web compare the radius of a positive ion to the radius of its neutral atom. Web the periodic law section 1 answer the following questions in the space provided. 2.) what can be removed from an atom is ionization energy is supplied. Web modern chemistry 37 the periodic law chapter 5 review the periodic law section 3 short answer answer the following questions in the space provided. Web 1) the modern definition of the atomic number 2) the atomic number is the basis for the organization of the periodic table, not atomic mass what did moseley's work lead to?
Web compare the radius of a positive ion to the radius of its neutral atom. Web modern chemistry 37 the periodic law chapter 5 review the periodic law section 3 short answer answer the following questions in the space provided. (b) according to mendeleev's original design. Web holt mcdougal modern chemistry 1 the periodic law chapter 5 review the periodic law section 2 short answer use this periodic table to answer the following questions in the space. _____ when an electron is added to a. The modern periodic law states that. Web the periodic law section 1 answer the following questions in the space provided. Based on the reasons and the arguments presented in the case, i am not surprised that wedow v. Web elements the elements in the modern periodic table are arranged according to increasing atomic number as a result of the work of henry moseley this arrangement is based on number of ___ in the nucleus of. The radius of a positive ion is smaller than the radius of its neutral atom.
Holt Chapter 5 (Periodic Table)
Web periodic law big idea the properties of the chemical elements are a periodic function of their atomic numbers. Web modern chemistry 37 the periodic law chapter 5 review the periodic law section 3 short answer answer the following questions in the space provided. Web holt mcdougal modern chemistry 1 the periodic law chapter 5 review the periodic law section.
Chemistry Periodic Table Study Guide Answers Periodic Table Timeline
Compare the radius of a negative ion to the radius of its neutral. In the early 1920s, virginia formed a. Web holt mcdougal modern chemistry 1 the periodic law chapter 5 review the periodic law section 2 short answer use this periodic table to answer the following questions in the space. 2.) what can be removed from an atom is.
The Periodic Table and Periodic Law Quiz Quizizz
The modern periodic law states that. What does the periodic law state? Web study with quizlet and memorize flashcards containing terms like the idea of arranging the elements in the periodic table according to their chemical and physical properties is attributed to, mendeleev left spaces in his periodic table and predicted several elements and their, in developing his periodic table,..
Atoms Elements And The Periodic Table Chapter Test Answer Key
(c) according to increasing atomic. Web terms in this set (10) 1.) an electron that is in the highest energy level of an atom's chemical properties is called an. Are you surprised that this is a 2006 case? (2 things) periodic law the physical and chemical properties of the elements are periodic functions of their atomic numbers periodic. Web holt.
PPT Chapter 5 The Periodic Law PowerPoint Presentation, free
Web 1) the modern definition of the atomic number 2) the atomic number is the basis for the organization of the periodic table, not atomic mass what did moseley's work lead to? Web study with quizlet and memorize flashcards containing terms like the idea of arranging the elements in the periodic table according to their chemical and physical properties is.
17+ Chapter 5 The Periodic Law RajinderRowan
Web the periodic law section 1 answer the following questions in the space provided. Compare the radius of a negative ion to the radius of its neutral. (c) according to increasing atomic. The physical and chemical properties of an element are functions of its atomic number. Periodic table review answer key.
HONORS Chapter 5 Test 2010.pdf Periodic Table Chemical Elements
In the early 1920s, virginia formed a. Web periodic law big idea the properties of the chemical elements are a periodic function of their atomic numbers. C in the modern periodic table, elements are ordered (a) according to decreasing atomic mass. Web study with quizlet and memorize flashcards containing terms like a person has a mass of 70.0 kg on.
Atoms Elements And The Periodic Table Chapter Test Answer Key
Web compare the radius of a positive ion to the radius of its neutral atom. Web elements the elements in the modern periodic table are arranged according to increasing atomic number as a result of the work of henry moseley this arrangement is based on number of ___ in the nucleus of. (b) according to mendeleev's original design. What does.
The Periodic Law
3.) across a period in the periodic. What does the periodic law state? Periodic table review answer key. 2.) what can be removed from an atom is ionization energy is supplied. Web terms in this set (10) 1.) an electron that is in the highest energy level of an atom's chemical properties is called an.
Organizing The Periodic Table Worksheet Answer Key / Periodic Table
The radius of a positive ion is smaller than the radius of its neutral atom. The discovery of the noble gases changed mendeleev's periodic table by. The building blocks of matter. Web periodic law big idea the properties of the chemical elements are a periodic function of their atomic numbers. Web elements the elements in the modern periodic table are.
Are You Surprised That This Is A 2006 Case?
(b) according to mendeleev's original design. Web terms in this set (10) 1.) an electron that is in the highest energy level of an atom's chemical properties is called an. The discovery of the noble gases changed mendeleev's periodic table by. In the early 1920s, virginia formed a.
What Does The Periodic Law State?
The physical and chemical properties of an element are functions of its atomic number. Web 1) the modern definition of the atomic number 2) the atomic number is the basis for the organization of the periodic table, not atomic mass what did moseley's work lead to? Based on the reasons and the arguments presented in the case, i am not surprised that wedow v. The radius of a positive ion is smaller than the radius of its neutral atom.
2.) What Can Be Removed From An Atom Is Ionization Energy Is Supplied.
Compare the radius of a negative ion to the radius of its neutral. Properties of elements repeat in a predictable way when atomic numbers are used to arrange elements into groups. Web the periodic law section 1 answer the following questions in the space provided. C in the modern periodic table, elements are ordered (a) according to decreasing atomic mass.
City Of Kansas City Case Is A 2006 Case.
This is because this case involve. Web study with quizlet and memorize flashcards containing terms like in the modern periodic table, elements are ordered, mendeleev noticed that certain similarities in the chemical properties of elements appeared at regular intervals when the elements were arranged in order of increasing, the modern periodic law. _____ when an electron is added to a. Web what did mendeleev's procedure do?