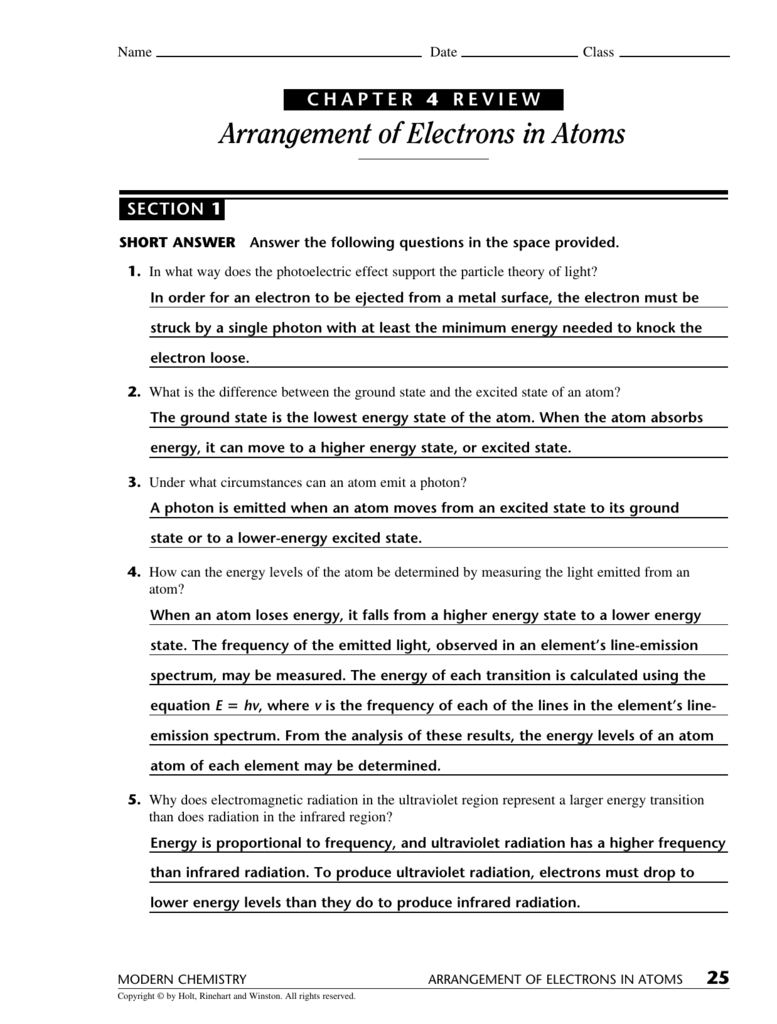
Chapter 4 Test Arrangement Of Electrons In Atoms Answer Key
Chapter 4 Test Arrangement Of Electrons In Atoms Answer Key - From the analysis of these results, the energy levels of an atom atom of each element. Other sets by this creator. When thinking about the different shaped paths on which electrons. Check out how easy it is to complete and esign documents online using fillable templates and a powerful editor. Which of the following orbital notations for. Electrons travel in pathways having four different shapes. Arrangement of electrons in atoms chemistry. The nucleus is at the center of an atom 2. Plays allison machac 4 years worksheet save share copy and edit. Electrons travel around the nucleus in specified energy levels.
Two of the valence electrons in the hcl molecule are shared, and the other six are located on the cl atom as lone pairs of electrons. 3, 2, 1, 0, 1, 2, 3 c. Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical relationship among the speed, wavelength, and frequency of electromagnetic radiation. On those energy levels, electrons. Electrons spin as they move along their pathways. Web accelerated chemistry 1 arrangement of electrons in atoms chapter 4 arrangement of electrons in atoms section 1 the development of a new atomic model objectives 1. Arrangement of electrons in atoms in the space provided, write the letter of the term that best completes each sentence or best answers each question. Web compare and contrast the bohr model and the quantum mechanical model of the atom. Bohr model says that electrons are in specific energy levels in orbits around the nucleus, the quantum mechanical model. In the format of 1s22s2, etc.
Did not have a scientific basis. Electrons cannot exist between those energy levels. Get everything done in minutes. Which of the following orbital notations for. Other sets by this creator. Electrons are found in layers around the nucleus of the atom. 3, 2, 1, 0, 1, 2, 3 c. As an electron moves from n=1 to n=4 energy is being. 1 d m 2 = 1 \mathrm{dm}^2= 1 dm 2 = m 2 \mathrm{m}^2 m 2. Click the card to flip 👆.
Chapter 4 electrons in atoms
3, 2, 1, 0, 1, 2, 3 c. Electrons cannot exist between those energy levels. Electrons spin as they move along their pathways. Web compare and contrast the bohr model and the quantum mechanical model of the atom. What is the total number of electrons needed to fill the fourth main energy level?
Atomic Structure Review Asnwer Key Atomic Structure And Chemical
The nucleus is at the center of an atom 2. Web accelerated chemistry 1 arrangement of electrons in atoms chapter 4 arrangement of electrons in atoms section 1 the development of a new atomic model objectives 1. Bohr model says that electrons are in specific energy levels in orbits around the nucleus, the quantum mechanical model. Plays allison machac 4.
Worksheets Electrons In Atoms Answers 3.12
1 d m 2 = 1 \mathrm{dm}^2= 1 dm 2 = m 2 \mathrm{m}^2 m 2. What are the possible angular momentum quantum numbers? Which of the following orbital notations for. What is the total number of electrons needed to fill the fourth main energy level? Web the principal quantum number of an electron is 4.
Chapter 5 Atomic Structure And The Periodic Table Answer Key
Claimed matter is made of atoms. Web two valence electrons per pb atom are transferred to cl atoms; Democritus’s original atomic theory was revised because it a. The resulting pb 2+ ion has a 6s 2 valence shell configuration. 0, 1, 2, 3 d.
Arrangement of Electrons in Atoms
Claimed atoms could be divided. Claimed matter is made of atoms. The nucleus is at the center of an atom 2. Electron configurations in the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical relationship.
Chapter 5 Electrons In Atoms Answers To Worksheet Promotiontablecovers
The statement that no two electrons. What are the possible angular momentum quantum numbers? Web two valence electrons per pb atom are transferred to cl atoms; The resulting pb 2+ ion has a 6s 2 valence shell configuration. When thinking about the different shaped paths on which electrons.
PPT Chapter 4 Arrangement of Electrons in Atoms PowerPoint
Web learn test match created by cschiavone130 honors chemistry mr. Electrons are found in 5 different shaped pathways. Democritus’s original atomic theory was revised because it a. 1 d m 2 = 1 \mathrm{dm}^2= 1 dm 2 = m 2 \mathrm{m}^2 m 2. Web compare and contrast the bohr model and the quantum mechanical model of the atom.
Chapter 4 electrons in atoms
In the format of 1s22s2, etc. Electrons are found in layers around the nucleus of the atom. Web describes mathematically the wave properties of electrons and other very small particles. 0, 1, 2, 3 d. Electrons travel in pathways having four different shapes.
Chapter 4 electrons in atoms
Web the principal quantum number of an electron is 4. Web arrangement of electrons in atoms chapter 4 review answer key. Web two valence electrons per pb atom are transferred to cl atoms; Bohr model says that electrons are in specific energy levels in orbits around the nucleus, the quantum mechanical model. 1 d m 2 = 1 \mathrm{dm}^2= 1.
Atomic Structure Worksheet Answer Key Label The Parts Of An Atom On The
From the analysis of these results, the energy levels of an atom atom of each element. Two of the valence electrons in the hcl molecule are shared, and the other six are located on the cl atom as lone pairs of electrons. Arrangement of electrons in atoms in the space provided, write the letter of the term that best completes.
Get Everything Done In Minutes.
3, 2, 1, 0, 1, 2, 3 c. Other sets by this creator. Click the card to flip 👆. Claimed matter is made of atoms.
Electrons Are Found In 5 Different Shaped Pathways.
The statement that no two electrons. Did not have a scientific basis. Democritus’s original atomic theory was revised because it a. Electrons cannot exist between those energy levels.
Arrangement Of Electrons In Atoms Chemistry.
Claimed atoms could be divided. Web describes mathematically the wave properties of electrons and other very small particles. The nucleus is at the center of an atom 2. Arrangement of electrons in atoms in the space provided, write the letter of the term that best completes each sentence or best answers each question.
Web Compare And Contrast The Bohr Model And The Quantum Mechanical Model Of The Atom.
Web the principal quantum number of an electron is 4. Click the card to flip 👆. Which of the following orbital notations for. Web learn test match created by cschiavone130 honors chemistry mr.