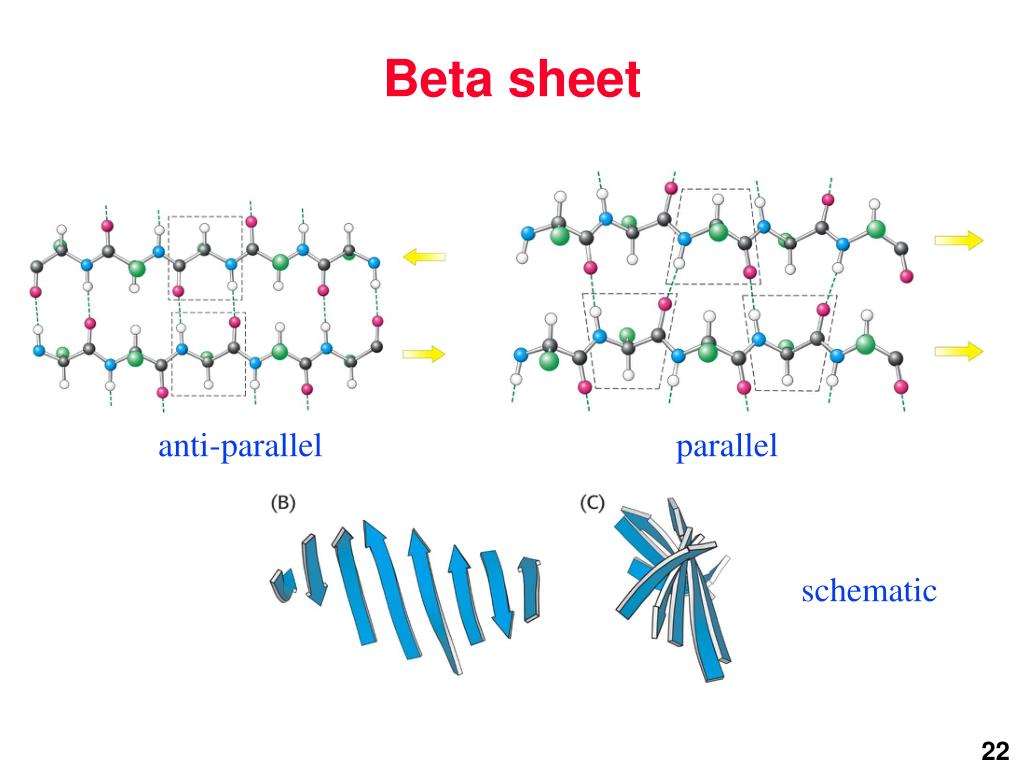
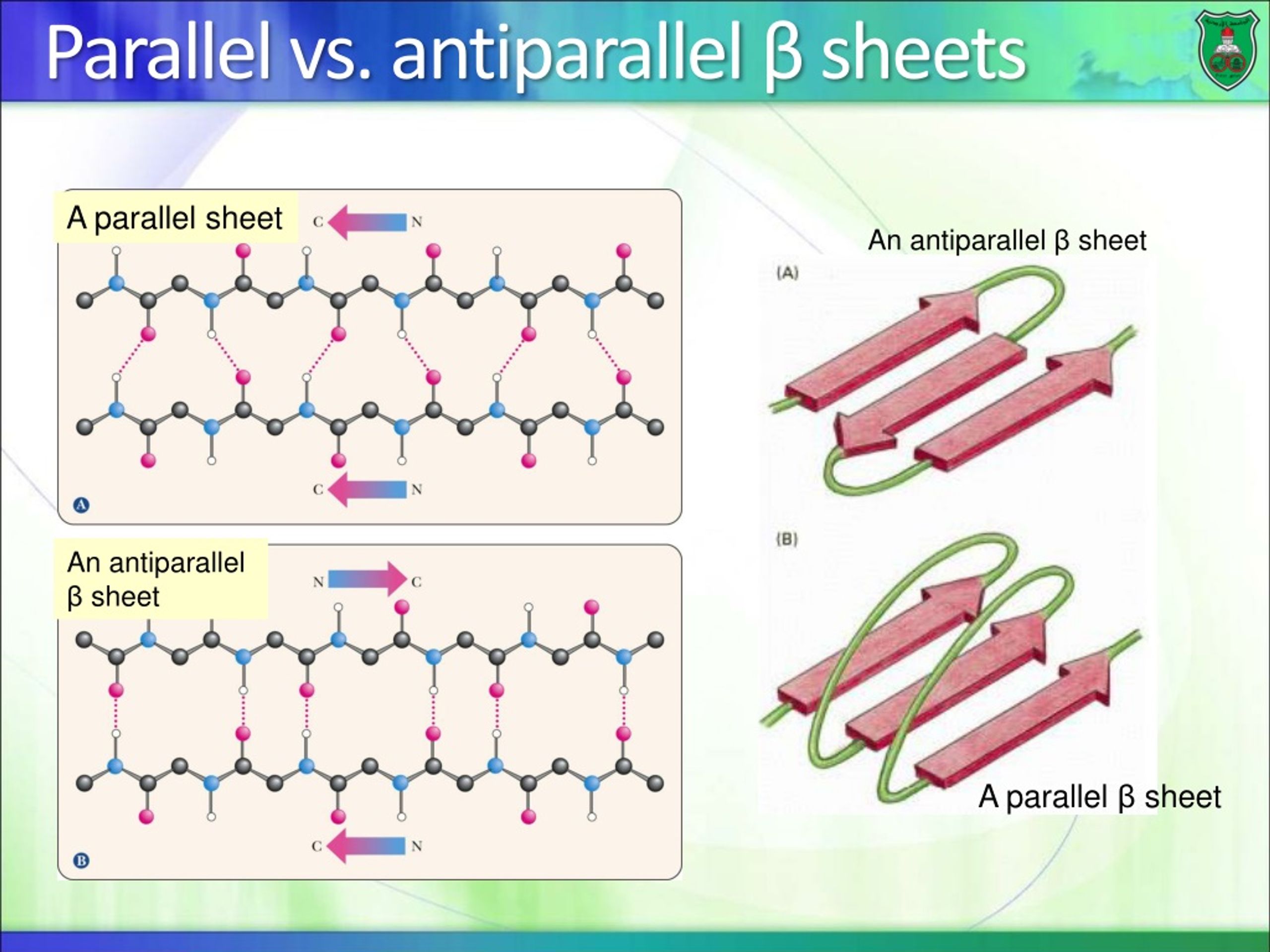
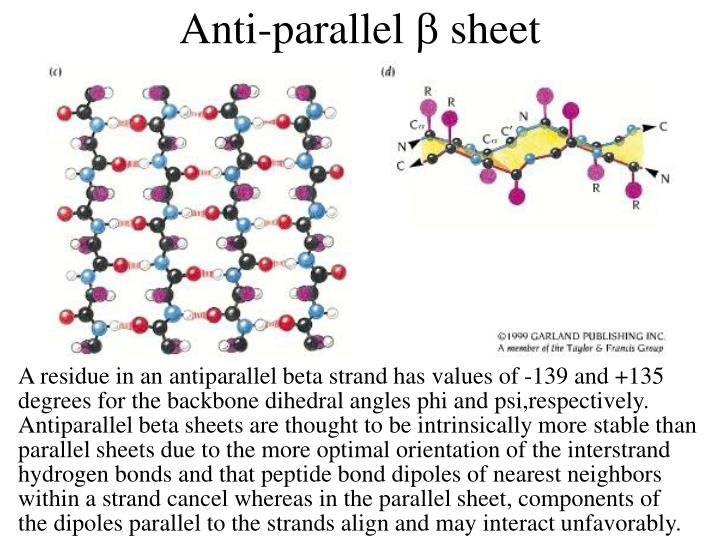
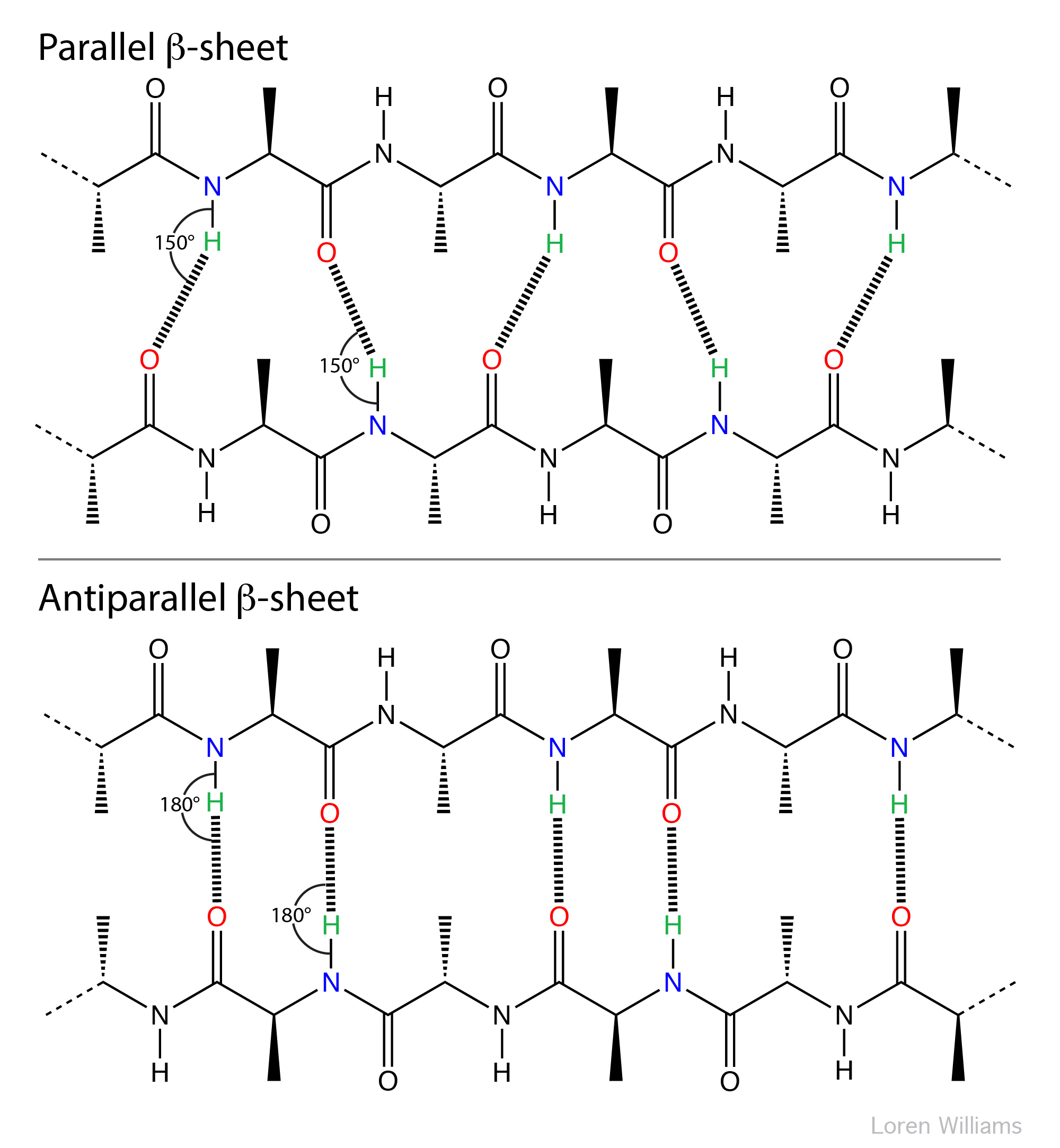
Anti Parallel Beta Sheet
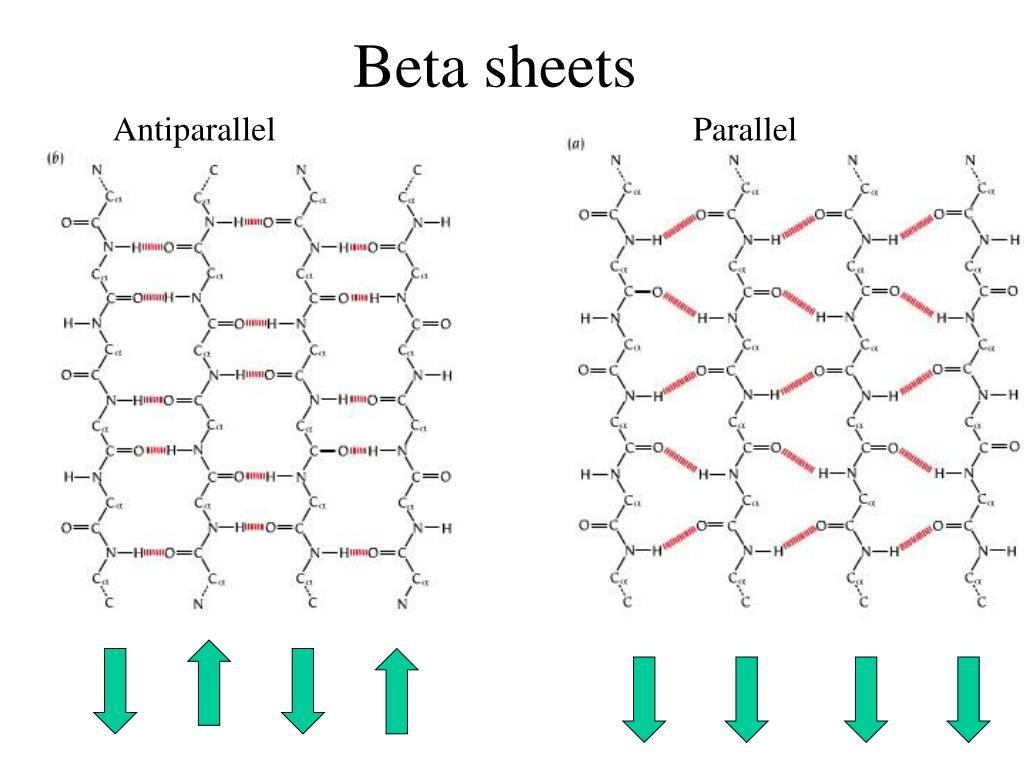
Anti Parallel Beta Sheet - Web the side chains in the beta sheet are normal to the plane of the sheet, extending out from the plane on alternating sides. Oxygen atoms are colored red and nitrogen atoms colored blue. Parallel sheets characteristically distribute hydrophobic side chains on both. Antiparallel ß sheets are slightly more. This can occur in the presence of two consecutive proline residues, which create an angled kink in.
Oxygen atoms are colored red and nitrogen atoms colored blue. Web the side chains in the beta sheet are normal to the plane of the sheet, extending out from the plane on alternating sides. Antiparallel ß sheets are slightly more. Parallel sheets characteristically distribute hydrophobic side chains on both. This can occur in the presence of two consecutive proline residues, which create an angled kink in.
Oxygen atoms are colored red and nitrogen atoms colored blue. Web the side chains in the beta sheet are normal to the plane of the sheet, extending out from the plane on alternating sides. This can occur in the presence of two consecutive proline residues, which create an angled kink in. Parallel sheets characteristically distribute hydrophobic side chains on both. Antiparallel ß sheets are slightly more.
PPT CENG 465 Introduction to Bioinformatics PowerPoint Presentation
Oxygen atoms are colored red and nitrogen atoms colored blue. Web the side chains in the beta sheet are normal to the plane of the sheet, extending out from the plane on alternating sides. Antiparallel ß sheets are slightly more. This can occur in the presence of two consecutive proline residues, which create an angled kink in. Parallel sheets characteristically.
PPT Protein structure PowerPoint Presentation, free download ID9193673
Parallel sheets characteristically distribute hydrophobic side chains on both. This can occur in the presence of two consecutive proline residues, which create an angled kink in. Oxygen atoms are colored red and nitrogen atoms colored blue. Web the side chains in the beta sheet are normal to the plane of the sheet, extending out from the plane on alternating sides..
PPT Secondary Structure Motifs of Proteins PowerPoint Presentation
Parallel sheets characteristically distribute hydrophobic side chains on both. This can occur in the presence of two consecutive proline residues, which create an angled kink in. Antiparallel ß sheets are slightly more. Web the side chains in the beta sheet are normal to the plane of the sheet, extending out from the plane on alternating sides. Oxygen atoms are colored.
Molecular Interactions (Noncovalent Interactions)
Oxygen atoms are colored red and nitrogen atoms colored blue. Parallel sheets characteristically distribute hydrophobic side chains on both. This can occur in the presence of two consecutive proline residues, which create an angled kink in. Antiparallel ß sheets are slightly more. Web the side chains in the beta sheet are normal to the plane of the sheet, extending out.
Parallel vs antiparallel betasheets YouTube
Antiparallel ß sheets are slightly more. Oxygen atoms are colored red and nitrogen atoms colored blue. Web the side chains in the beta sheet are normal to the plane of the sheet, extending out from the plane on alternating sides. This can occur in the presence of two consecutive proline residues, which create an angled kink in. Parallel sheets characteristically.
Structure of residues 1540 in antiparallel D23NAβ 140 (SFg2
This can occur in the presence of two consecutive proline residues, which create an angled kink in. Antiparallel ß sheets are slightly more. Parallel sheets characteristically distribute hydrophobic side chains on both. Web the side chains in the beta sheet are normal to the plane of the sheet, extending out from the plane on alternating sides. Oxygen atoms are colored.
antiparallel beta sheet NIH 3D Print Exchange
Oxygen atoms are colored red and nitrogen atoms colored blue. Web the side chains in the beta sheet are normal to the plane of the sheet, extending out from the plane on alternating sides. Antiparallel ß sheets are slightly more. This can occur in the presence of two consecutive proline residues, which create an angled kink in. Parallel sheets characteristically.
parallel beta sheet Cheaper Than Retail Price> Buy Clothing
Web the side chains in the beta sheet are normal to the plane of the sheet, extending out from the plane on alternating sides. Parallel sheets characteristically distribute hydrophobic side chains on both. Oxygen atoms are colored red and nitrogen atoms colored blue. Antiparallel ß sheets are slightly more. This can occur in the presence of two consecutive proline residues,.
PPT Introduction to Protein Structure PowerPoint Presentation, free
Web the side chains in the beta sheet are normal to the plane of the sheet, extending out from the plane on alternating sides. Oxygen atoms are colored red and nitrogen atoms colored blue. Antiparallel ß sheets are slightly more. This can occur in the presence of two consecutive proline residues, which create an angled kink in. Parallel sheets characteristically.
Difference in preferred amino acids for parallel vs. antiparallel beta
Parallel sheets characteristically distribute hydrophobic side chains on both. Oxygen atoms are colored red and nitrogen atoms colored blue. Antiparallel ß sheets are slightly more. Web the side chains in the beta sheet are normal to the plane of the sheet, extending out from the plane on alternating sides. This can occur in the presence of two consecutive proline residues,.
Web The Side Chains In The Beta Sheet Are Normal To The Plane Of The Sheet, Extending Out From The Plane On Alternating Sides.
This can occur in the presence of two consecutive proline residues, which create an angled kink in. Parallel sheets characteristically distribute hydrophobic side chains on both. Oxygen atoms are colored red and nitrogen atoms colored blue. Antiparallel ß sheets are slightly more.