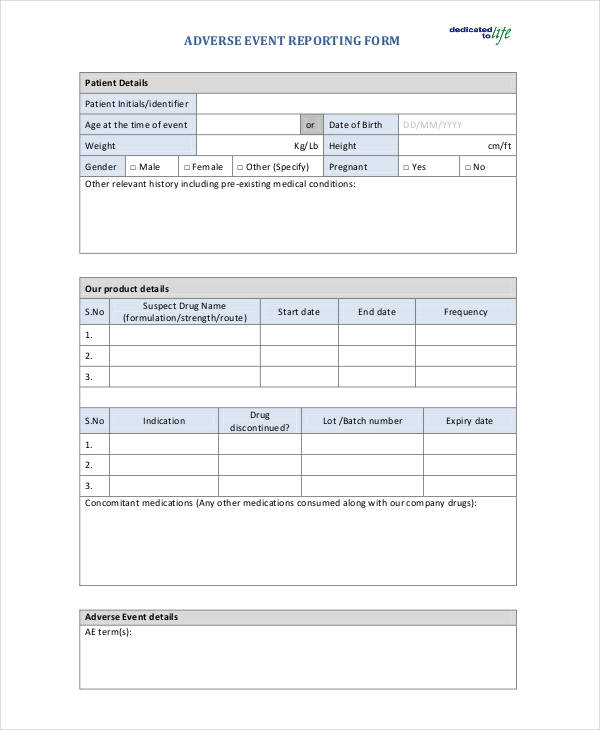
Adverse Event Reporting Form
Adverse Event Reporting Form - Web a vaccine adverse event reporting form is a document that is filled by a patient to report an adverse event related to an immunization, usually given for medical purposes to. Clinic use (for transmission from clinic to the cc and nhlbi) 1. Was this an unexpected adverse event? At study site or elsewhere): Web medwatch is the food and drug administration's (fda) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure,. Web fda use only triage unit u.s. Web 1 of 2 30apr2020 serious adverse event (sae)page 1 of 2 30apr2020 serious adverse event (sae) report form study name protocol number: Web adverse event reporting form adverse events are defined as incidents that have a direct or indirect impact on the community, patients, staff, and/or the sud treatment. Web adverse event report form. Department of health and human services foodand drug administrationmedwatch form fda 3500 (2/19) (continued) the fda safety.
Web serious adverse event report form(s) to the cc and nhlbi. Web 1 of 2 30apr2020 serious adverse event (sae)page 1 of 2 30apr2020 serious adverse event (sae) report form study name protocol number: Web adverse event reporting form adverse events are defined as incidents that have a direct or indirect impact on the community, patients, staff, and/or the sud treatment. Brief description of participant with no personal. The fda safety information and adverse event reporting program medwatch, the fda’s medical product safety reporting program for health. This form must be completed and submitted to the ddd case manager. If for some reason an adverse event report is made about an event not listed in items 1 through 27 above, a brief description of the event should be included on this. Web adverse event reporting form please submit all cases within 1 working day of receipt of report submission of a report does not constitute an admission that medical personnel,. At study site or elsewhere): Web adverse event form clinical trial.
Clinic use (for transmission from clinic to the cc and nhlbi) 1. Web a vaccine adverse event reporting form is a document that is filled by a patient to report an adverse event related to an immunization, usually given for medical purposes to. Department of health and human services foodand drug administrationmedwatch form fda 3500 (2/19) (continued) the fda safety. Web adverse event reporting form adverse events are defined as incidents that have a direct or indirect impact on the community, patients, staff, and/or the sud treatment. Use this form to record the specific events “as is” without compromising. Location of serious adverse event (e.g. At study site or elsewhere): Web adverse event reporting form please submit all cases within 1 working day of receipt of report submission of a report does not constitute an admission that medical personnel,. Web medwatch is the food and drug administration's (fda) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure,. Web fda use only triage unit u.s.
Adapted from current adverse event reporting guidelines under
Web adverse event form clinical trial. Online reporting (i.e., electronic form) is strongly encouraged. The fda safety information and adverse event reporting program medwatch, the fda’s medical product safety reporting program for health. This form must be completed and submitted to the ddd case manager. Location of serious adverse event (e.g.
Serious Adverse Event Form Template SampleTemplatess SampleTemplatess
Clinic use (for transmission from clinic to the cc and nhlbi) 1. Use this form to record the specific events “as is” without compromising. The fda safety information and adverse event reporting program medwatch, the fda’s medical product safety reporting program for health. Web 1 of 2 30apr2020 serious adverse event (sae)page 1 of 2 30apr2020 serious adverse event (sae).
FREE 37+ Event Forms in PDF Excel MS Word
Location of serious adverse event (e.g. If for some reason an adverse event report is made about an event not listed in items 1 through 27 above, a brief description of the event should be included on this. Web adverse event form clinical trial. Web adverse event reporting form adverse events are defined as incidents that have a direct or.
New Mexico Adverse Events Reporting Form Download Fillable PDF
Web fda use only triage unit u.s. 01/18) this form must be completed within 72 hours of the adverse event. Was this an unexpected adverse event? Department of health and human services foodand drug administrationmedwatch form fda 3500 (2/19) (continued) the fda safety. The fda safety information and adverse event reporting program medwatch, the fda’s medical product safety reporting program.
Massachusetts Serious Adverse Event Report Form Download Printable PDF
Please type or write legibly. Web serious adverse event report form(s) to the cc and nhlbi. This form must be completed and submitted to the ddd case manager. Clinic use (for transmission from clinic to the cc and nhlbi) 1. Department of health and human services foodand drug administrationmedwatch form fda 3500 (2/19) (continued) the fda safety.
ADR reporting form. Download Scientific Diagram
Web adverse event form clinical trial. Web serious adverse event report form(s) to the cc and nhlbi. Web medwatch is the food and drug administration's (fda) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure,. At study site or elsewhere): Clinic use (for transmission from clinic to the cc and nhlbi) 1.
FREE 37+ Event Forms in PDF Excel MS Word
Online reporting (i.e., electronic form) is strongly encouraged. At study site or elsewhere): Use this form to record the specific events “as is” without compromising. Web adverse event reporting form please submit all cases within 1 working day of receipt of report submission of a report does not constitute an admission that medical personnel,. Web serious adverse event report form(s).
FREE 11+ Adverse Event Forms in PDF MS Word Excel
Web a vaccine adverse event reporting form is a document that is filled by a patient to report an adverse event related to an immunization, usually given for medical purposes to. At study site or elsewhere): Within 72 hours of the adverse. Web 1 of 2 30apr2020 serious adverse event (sae)page 1 of 2 30apr2020 serious adverse event (sae) report.
FREE 33+ Event Forms in PDF MS Word Excel
Use this form to record the specific events “as is” without compromising. Web adverse event reporting form please submit all cases within 1 working day of receipt of report submission of a report does not constitute an admission that medical personnel,. This form must be completed and submitted to the ddd case manager. Web adverse event reporting form adverse events.
Serious Adverse events reporting form
This form must be completed and submitted to the ddd case manager. Was this an unexpected adverse event? Online reporting (i.e., electronic form) is strongly encouraged. Web a vaccine adverse event reporting form is a document that is filled by a patient to report an adverse event related to an immunization, usually given for medical purposes to. Web serious adverse.
The Fda Safety Information And Adverse Event Reporting Program Medwatch, The Fda’s Medical Product Safety Reporting Program For Health.
Web adverse event report form. Please type or write legibly. Web fda use only triage unit u.s. Location of serious adverse event (e.g.
This Form Must Be Completed And Submitted To The Ddd Case Manager.
Use this form to record the specific events “as is” without compromising. Web serious adverse event report form(s) to the cc and nhlbi. Brief description of participant with no personal. Web medwatch is the food and drug administration's (fda) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure,.
Clinic Use (For Transmission From Clinic To The Cc And Nhlbi) 1.
Department of health and human services foodand drug administrationmedwatch form fda 3500 (2/19) (continued) the fda safety. Web adverse event reporting form adverse events are defined as incidents that have a direct or indirect impact on the community, patients, staff, and/or the sud treatment. Was this an unexpected adverse event? Web a vaccine adverse event reporting form is a document that is filled by a patient to report an adverse event related to an immunization, usually given for medical purposes to.
Within 72 Hours Of The Adverse.
At study site or elsewhere): Online reporting (i.e., electronic form) is strongly encouraged. 01/18) this form must be completed within 72 hours of the adverse event. Web 1 of 2 30apr2020 serious adverse event (sae)page 1 of 2 30apr2020 serious adverse event (sae) report form study name protocol number: